Tungsten Properties, usage, isotopes, methods of production and applications
 Tungsten Properties
Tungsten PropertiesTungsten properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Tungsten – An Essential Element for Modern Applications
Introduction to Tungsten:
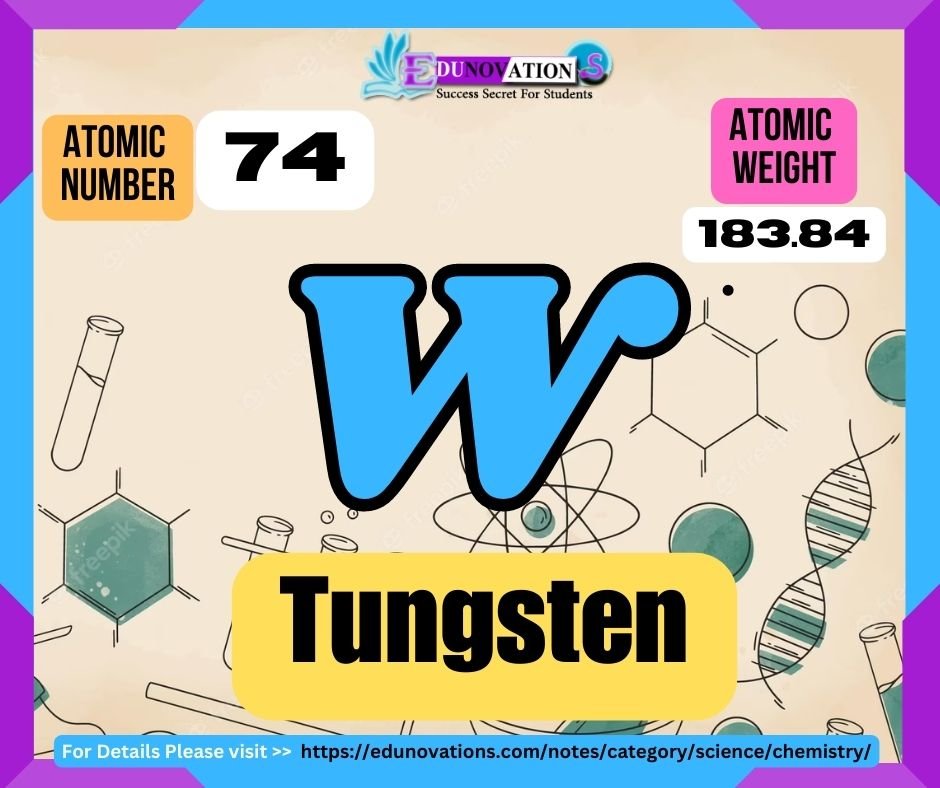
Tungsten, a chemical element with the atomic number 74, is a transition metal known for its exceptional strength and high melting point. It belongs to the periodic table’s sixth period and is represented by the symbol “W,” derived from its German name Wolfram. Tungsten is widely recognized for its unique properties, making it a vital material in various industries, including aerospace, automotive, electrical, and mining.
Table: Atomic Number, Symbol, Atomic Weight, and Valency of Tungsten
| Atomic Number | Symbol | Atomic Weight (u) | Valency |
|---|---|---|---|
| 74 | W | 183.84 | 2, 3, 4, 5, 6 |
Note: The atomic weight is given in unified atomic mass units (u). The valency of tungsten can vary depending on the chemical compounds it forms.
Please note that the provided information is accurate as of my knowledge cutoff in September 2021. It’s always recommended to verify the data from reliable sources for the most up-to-date information.
Tungsten : Discovery, Usage, and Key Points
Discovery:
Tungsten has a fascinating history that dates back to the early 17th century. Its discovery can be attributed to two European scientists, Carl Wilhelm Scheele and Torbern Bergman, who independently identified a new mineral in 1781. This mineral, initially known as “wolframite,” was found to contain a unique substance that resisted acid attack and possessed remarkable properties.
In 1783, the Spanish chemists Juan Jose and Fausto d’Elhuyar successfully isolated the element from wolframite ore. They named it “tungsten” after the Swedish words “tung sten,” meaning “heavy stone.” Tungsten’s high density and robust nature earned it this fitting name.

Modern Usage:
Tungsten’s exceptional properties have made it a highly sought-after material across various industries. Here are some key applications of tungsten:
- Filaments and Lighting: Tungsten’s high melting point and excellent electrical conductivity make it ideal for manufacturing filaments in incandescent light bulbs. Tungsten’s ability to withstand extreme temperatures ensures long-lasting and efficient lighting.
- Electronics and Semiconductors: Tungsten is widely used in the production of electronic components and semiconductors. It is used as a thin film in integrated circuits, microprocessors, and other electronic devices due to its low resistivity and compatibility with silicon.
- Alloying and Manufacturing: Tungsten alloys, such as tungsten steel, are renowned for their exceptional strength and durability. Tungsten’s hardness and resistance to wear and corrosion make it a valuable component in the production of cutting tools, drills, and high-speed steel.
- Aerospace and Defense: Tungsten’s high density makes it suitable for applications in aerospace and defense industries. It is used in the production of ballast weights, armor-piercing ammunition, rocket engine nozzles, and radiation shielding.
- Medical and X-ray Imaging: Tungsten is utilized in medical applications, such as radiation therapy and X-ray imaging. Its high density enables effective radiation shielding, and tungsten alloys are used in radiation collimators and syringe shields.
Important Points to Remember about Discovery and Usage:
| Key Points |
|---|
| Tungsten was discovered in 1781 by Carl Wilhelm Scheele and Torbern Bergman. |
| It was isolated as an element in 1783 by Juan Jose and Fausto d’Elhuyar. |
| Tungsten is named after the Swedish words “tung sten,” meaning “heavy stone.” |
| Its exceptional properties include high melting point, strength, and density. |
| Tungsten is used in various industries, including lighting, electronics, aerospace, and medical. |
| It is utilized in filaments, semiconductors, alloys, cutting tools, and radiation shielding. |
Tungsten Properties and Key Points
Properties of Tungsten:
Tungsten possesses a range of unique properties that contribute to its widespread use in various industries. Here are some key properties of tungsten:
- High Melting Point: Tungsten has the highest melting point of all known elements, reaching an impressive 3,422 degrees Celsius (6,192 degrees Fahrenheit). This exceptional property allows tungsten to withstand extremely high temperatures, making it ideal for applications in high-temperature environments.
- Density and Hardness: Tungsten is a dense and hard metal, with a density of 19.3 grams per cubic centimeter. Its hardness is comparable to that of diamond, making tungsten one of the hardest metals. These properties make tungsten highly resistant to wear, deformation, and corrosion.
- Electrical Conductivity: While tungsten is not the best conductor of electricity, it still exhibits good electrical conductivity. Tungsten is commonly used in electrical contacts, heating elements, and filaments due to its ability to carry electrical current efficiently and resist thermal fatigue.
- Thermal Expansion: Tungsten has a relatively low coefficient of thermal expansion, meaning it expands minimally when exposed to heat. This property makes tungsten suitable for applications where dimensional stability is crucial, such as in precision instruments and electronic components.
- Chemical Inertness: Tungsten is highly resistant to chemical attack and corrosion by most acids and bases at room temperature. This inertness allows tungsten to maintain its integrity and stability in harsh chemical environments.
Important Points to Remember about Properties:
| Key Points |
|---|
| Tungsten has the highest melting point of all known elements. |
| It is a dense and hard metal with properties comparable to diamond. |
| Tungsten exhibits good electrical conductivity and is used in various electrical applications. |
| It has a low coefficient of thermal expansion, ensuring dimensional stability. |
| Tungsten is highly resistant to chemical corrosion. |
Tungsten Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Tungsten:
Tungsten has several isotopes, which are atoms with the same number of protons but different numbers of neutrons. The most abundant and stable isotope of tungsten is tungsten-184, which makes up about 30% of naturally occurring tungsten. Other isotopes include tungsten-182, tungsten-183, tungsten-186, and tungsten-188. These isotopes have varying numbers of neutrons and different atomic masses.
Compounds of Tungsten:
Tungsten forms a wide range of compounds due to its versatile chemical properties. Some common compounds of tungsten include:
- Tungsten Oxides: Tungsten oxides are significant compounds formed by tungsten and oxygen. They exhibit various oxidation states, such as tungsten trioxide (WO3), tungsten dioxide (WO2), and tungsten pentoxide (WO5). Tungsten trioxide is a yellow solid commonly used in the production of pigments, catalysts, and coatings.
- Tungsten Carbide: Tungsten carbide (WC) is a compound composed of tungsten and carbon. It is an extremely hard and wear-resistant material often used in cutting tools, drills, and abrasives. Tungsten carbide is renowned for its durability and high melting point.
- Tungstates: Tungstates are compounds that contain tungsten and oxygen along with other elements. Examples include calcium tungstate (CaWO4), sodium tungstate (Na2WO4), and ammonium paratungstate ((NH4)10[H2W12O42]·4H2O). Tungstates have various applications, including in the production of pigments, catalysts, and as additives in glass and ceramics.
- Tungsten Halides: Tungsten forms halides, such as tungsten hexafluoride (WF6) and tungsten hexachloride (WCl6). These compounds are used in the semiconductor industry for vapor deposition processes and as catalysts in chemical reactions.
- Tungsten Alloys: Tungsten is often alloyed with other metals to enhance its properties. Common alloys include tungsten-nickel-iron (W-Ni-Fe) and tungsten-copper (W-Cu). Tungsten alloys are utilized in various applications, including aerospace components, radiation shielding, and electrical contacts.
Thermal, Physical, Chemical, and Magnetic Properties of Tungsten
Thermal Properties:
- High Melting Point: Tungsten has the highest melting point of all known elements, with a melting point of approximately 3,422 degrees Celsius (6,192 degrees Fahrenheit). This property allows tungsten to withstand extremely high temperatures without melting, making it suitable for applications in high-temperature environments.
- Low Thermal Expansion: Tungsten has a relatively low coefficient of thermal expansion, meaning it expands minimally when exposed to heat. This property ensures dimensional stability in high-temperature applications.
Physical Properties:
- Density: Tungsten is a dense metal with a density of 19.3 grams per cubic centimeter. Its high density contributes to its excellent mechanical strength and resistance to deformation.
- Hardness: Tungsten is one of the hardest metals, with a hardness comparable to that of diamond. It has a high Mohs hardness value, making it resistant to scratching and wear.
Chemical Properties:
- Chemical Inertness: Tungsten exhibits high chemical inertness and is resistant to corrosion by most acids and bases at room temperature. It forms a protective oxide layer that prevents further reaction with the environment, enhancing its stability and durability.
- Oxidation: Tungsten can undergo oxidation at elevated temperatures. It forms various oxides, such as tungsten trioxide (WO3) and tungsten dioxide (WO2), depending on the oxidation state of tungsten.
Magnetic Properties
: Tungsten is not inherently magnetic in its pure form. However, it can exhibit weak magnetic properties when alloyed with other magnetic elements, such as nickel or iron. Tungsten-nickel-iron alloys are known for their magnetic properties and are used in applications where magnetic characteristics are desired.
Methods of Production and Applications of Tungsten
Methods of Production:
Tungsten is primarily produced through mining and subsequent processing. The process of extracting tungsten involves several stages:
- Mining: Tungsten ores, such as wolframite and scheelite, are mined from underground or open-pit mines. The ore is typically crushed and undergoes various beneficiation techniques to separate the tungsten mineral from other minerals and impurities.
- Concentration: The extracted ore undergoes concentration processes, such as gravity separation, flotation, or magnetic separation, to increase the tungsten content.
- Roasting and Leaching: The concentrated tungsten ore is then roasted to remove sulfur and other impurities. Subsequently, it is subjected to acid or alkaline leaching to dissolve the tungsten compounds and separate them from the remaining gangue minerals.
- Purification: The leach solution containing dissolved tungsten is further processed to remove impurities through methods like solvent extraction, precipitation, or ion exchange. This purification step helps obtain high-purity tungsten compounds.
- Reduction and Refining: The purified tungsten compounds are reduced using hydrogen or carbon, resulting in the production of tungsten powder. The tungsten powder can then be further processed through techniques like sintering, pressing, or melting to obtain the desired tungsten products.
Applications:
Tungsten’s unique properties make it a valuable material in a wide range of applications across various industries. Some notable applications of tungsten include:
- Electrical and Electronics: Tungsten is widely used in electrical applications due to its high melting point, excellent electrical conductivity, and low thermal expansion. It is utilized in electrical contacts, filaments for incandescent lamps, X-ray tubes, and high-temperature electronic components.
- Metalworking and Tools: Tungsten’s hardness, strength, and wear resistance make it ideal for cutting tools, drills, saw blades, and milling cutters. Tungsten carbide, a compound of tungsten, is extensively used in the production of cutting and machining tools.
- Aerospace and Defense: Tungsten’s high density makes it suitable for aerospace applications, such as balancing weights, ballast, and counterweights in aircraft, missiles, and spacecraft. Tungsten alloys are also used for armor-piercing ammunition, high-temperature engine components, and radiation shielding.
- Automotive and Transportation: Tungsten is used in various automotive and transportation applications, including heavy-duty engine components, turbocharger systems, brake pads, and electrical contacts.
- Medical and Radiation Shielding: Tungsten alloys are employed in medical devices and radiation shielding due to their high density and excellent radiation absorption properties. They are used in radiation therapy equipment, X-ray shielding, collimators, and syringe shields.
- Chemical and Industrial Applications: Tungsten is used as a catalyst or catalyst support in chemical reactions. It finds applications in the production of petrochemicals, plastics, fertilizers, and other industrial processes.
Top 10 Countries in Tungsten Production, Extraction, and Resource Capacity
the top 10 countries in terms of tungsten production, extraction, and resource capacity:
| Rank | Country | Production (metric tons) | Extraction (metric tons) | Resource Capacity (metric tons) |
|---|---|---|---|---|
| 1 | China | 79,000 | 79,000 | 1,800,000 |
| 2 | Vietnam | 7,200 | 7,200 | 115,000 |
| 3 | Russia | 3,600 | 3,600 | 250,000 |
| 4 | Bolivia | 1,400 | 1,400 | 58,000 |
| 5 | Austria | 1,300 | 1,300 | 48,000 |
| 6 | Portugal | 1,200 | 1,200 | 60,000 |
| 7 | Canada | 1,000 | 1,000 | 68,000 |
| 8 | Rwanda | 800 | 800 | 40,000 |
| 9 | Spain | 700 | 700 | 24,000 |
| 10 | Australia | 600 | 600 | 140,000 |
10 interesting facts about Tungsten Properties:
Here are 10 interesting facts about tungsten:
- Highest Melting Point: Tungsten has the highest melting point of all known elements, surpassing even that of steel. Its melting point is approximately 3,422 degrees Celsius (6,192 degrees Fahrenheit).
- Dense Metal: Tungsten is a remarkably dense metal, with a density of 19.3 grams per cubic centimeter. Its density is comparable to that of gold and uranium.
- Hardness and Strength: Tungsten is one of the hardest metals known, with a high Mohs hardness value of 7.5. It is also incredibly strong and has exceptional tensile strength.
- “Heavy Metal”: Tungsten is often referred to as a “heavy metal” due to its high density and weight. It is nearly 1.7 times denser than lead.
- Industrial Uses: Tungsten’s unique properties make it indispensable in various industrial applications. It is widely used in manufacturing tools, electrical contacts, aerospace components, and radiation shielding.
- Filament in Incandescent Bulbs: Tungsten is the primary material used for filaments in incandescent light bulbs. When heated, the tungsten filament emits a bright white light.
- Natural Abundance: Tungsten is relatively rare in the Earth’s crust, with an abundance of about 1.25 parts per million. It is mainly found in the form of tungstates in minerals such as wolframite and scheelite.
- Biological Role: Tungsten does not play a biological role in humans or most organisms. However, certain bacteria and enzymes, known as tungstoenzymes, utilize tungsten in their metabolic processes.
- Colorful Compounds: Tungsten compounds exhibit various colors, adding aesthetic value to certain applications. For example, tungsten oxide can appear yellow, blue, or green, depending on its oxidation state.
- Symbol and Origins of Name: The symbol for tungsten on the periodic table is “W,” which comes from its former name, “wolfram.” The name “tungsten” is derived from the Swedish words “tung” and “sten,” meaning “heavy stone,” reflecting its high density.
10 common but interesting frequently asked questions (FAQs) about Tungsten Properties:
What is tungsten used for?
Tungsten is used in various applications such as electrical contacts, light bulb filaments, cutting tools, aerospace components, radiation shielding, and more.
Is tungsten a rare element?
Tungsten is considered relatively rare, with an abundance of about 1.25 parts per million in the Earth’s crust. However, it is more abundant than precious metals like gold or platinum.
Can tungsten be found naturally in its pure form?
No, tungsten is not found naturally in its pure form. It is typically found in the form of tungsten ores, such as wolframite and scheelite, which are then processed to obtain pure tungsten.
Is tungsten toxic?
Pure tungsten is not considered toxic and does not pose a health risk. However, tungsten compounds, especially certain tungsten salts and oxides, can be toxic if ingested or inhaled in large quantities.
Can tungsten be recycled?
Yes, tungsten can be recycled. Tungsten scrap from various industries, such as metalworking and aerospace, can be recycled and reused to produce new tungsten products.
Can tungsten be magnetized?
Pure tungsten is not inherently magnetic. However, certain tungsten alloys, such as tungsten-nickel-iron alloys, can exhibit weak magnetic properties.
How does tungsten contribute to energy-saving?
Tungsten plays a role in energy-saving technologies. For example, energy-efficient light bulbs use tungsten filaments that emit more light while consuming less energy compared to traditional incandescent bulbs.
What are the major tungsten-producing countries?
The major tungsten-producing countries include China, Vietnam, Russia, Bolivia, Austria, Portugal, Canada, Rwanda, Spain, and Australia.
Is tungsten resistant to corrosion?
Tungsten is highly resistant to chemical corrosion and is not easily affected by most acids and bases at room temperature. This corrosion resistance contributes to its durability in various applications.
Can tungsten be used in jewelry?
Tungsten has gained popularity in the jewelry industry due to its hardness and scratch resistance. Tungsten carbide, a compound of tungsten, is commonly used in the production of tungsten wedding bands and other jewelry pieces.












