Protactinium Properties, usage, isotopes, methods of production and applications
 Protactinium Properties
Protactinium PropertiesProtactinium properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Protactinium – An Essential Element for Modern Applications
Introduction: Protactinium is a chemical element with the symbol Pa and atomic number 91. It is a rare and highly radioactive metal that belongs to the actinide series of elements. The element was first discovered in 1913 by two scientists, Kasimir Fajans and Otto Gohring, who named it “protactinium” due to its position preceding the element actinium in the periodic table.
Protactinium is a silvery-gray metal that tarnishes slowly when exposed to air. It is highly reactive and readily forms chemical compounds with other elements. Due to its rarity and radioactivity, protactinium has limited practical applications. However, it has been used in scientific research and in nuclear reactors for studying nuclear physics and as a precursor to the production of the element uranium-233, which can be used as nuclear fuel.
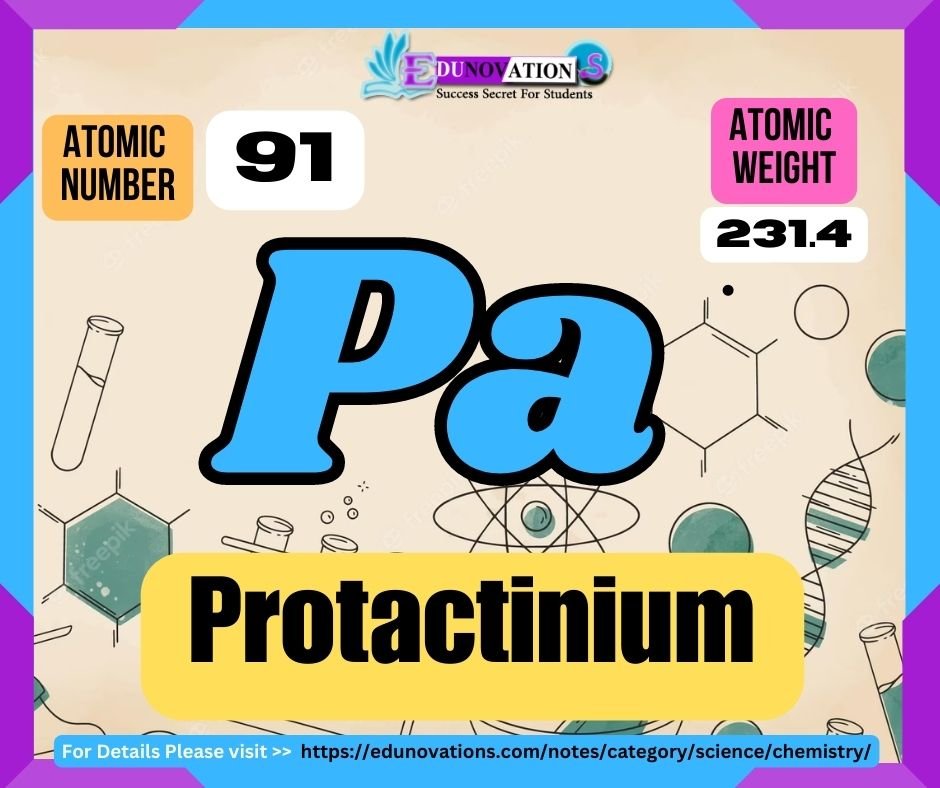
Table: Atomic Number, Symbol, Atomic Weight, and Valency of Protactinium
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 91 | Pa | 231.03588 | +5 |
Note: The atomic weight mentioned in the table represents the standard atomic weight of naturally occurring protactinium, which includes various isotopes with different atomic masses. Valency indicates the common oxidation state of protactinium, primarily found as Pa(V).
By utilizing this table and information, readers can quickly grasp the essential details about protactinium, including its atomic number, symbol, atomic weight, and valency.
Protactinium : Discovery, Usage, and Key Points
Discovery:
Protactinium was first discovered in 1913 by two scientists, Kasimir Fajans and Otto Gohring. They identified a radioactive substance that appeared before actinium in the periodic table and named it “protactinium,” which means “precursor to actinium.” The discovery of protactinium played a significant role in expanding our understanding of the periodic table and the nature of radioactive elements.

Modern Usage:
- Nuclear Research: Protactinium has been primarily used in scientific research, particularly in the field of nuclear physics. Its radioactive properties make it useful for studying the behavior of atoms and subatomic particles. It has contributed to advancements in understanding nuclear reactions and the structure of atomic nuclei.
- Nuclear Reactors: Protactinium-233, a radioisotope of protactinium, can be used as a precursor for the production of uranium-233. Uranium-233 is a fissile material that can sustain a nuclear chain reaction, making it potentially valuable as a fuel in nuclear reactors. However, due to its limited availability and challenging extraction process, the practical application of protactinium in this context remains limited.
- Isotope Dating: Protactinium-231, another isotope of protactinium, has a half-life of approximately 32,500 years. This characteristic has been utilized in the field of radiometric dating to determine the age of geological samples and materials.
- Scientific Instrumentation: Protactinium and its compounds have been employed in the production of specialized scientific instruments, including radiation detectors and spectrometers. These instruments play a crucial role in various scientific disciplines such as nuclear physics, environmental monitoring, and materials science.
Important Points to Remember about Discovery and Usage:
| Key Points |
|---|
| Protactinium was discovered in 1913 by Fajans and Gohring. |
| It is primarily used in scientific research and nuclear physics. |
| Protactinium-233 can be used as a precursor to uranium-233. |
| Protactinium-231 is used in radiometric dating. |
| It finds applications in scientific instrumentation. |
Protactinium Properties and Key Points
Properties:
- Physical State: Protactinium is a silvery-gray, lustrous metal at room temperature. It is solid and has a high density, making it one of the densest elements known.
- Radioactivity: Protactinium is highly radioactive, with all its isotopes being unstable. Its most stable isotope, protactinium-231, has a half-life of approximately 32,500 years. This radioactivity necessitates careful handling and containment.
- Chemical Reactivity: Protactinium is highly reactive, especially when exposed to air, water, and acids. It readily reacts with oxygen to form a protective oxide layer, which slows down further oxidation. However, prolonged exposure to air can lead to the formation of a black tarnish.
- Valency: The most common oxidation state of protactinium is +5. In this state, it forms compounds with various elements, such as halides, oxides, and sulfides.
- Nuclear Properties: Protactinium is a key element in the thorium-uranium fuel cycle. Through a series of radioactive decays, it can produce uranium-233, a fissile isotope that can be used as fuel in nuclear reactors.
- Isotopes: Protactinium has several isotopes, including protactinium-231, protactinium-233, and protactinium-234. These isotopes differ in their stability and decay properties, contributing to the element’s radioactivity.
Important Points to Remember about Properties:
| Key Points |
|---|
| Protactinium is a highly radioactive metal. |
| It forms a protective oxide layer when exposed to air. |
| Protactinium primarily exhibits a +5 oxidation state. |
| It plays a role in the thorium-uranium fuel cycle. |
| Protactinium has various isotopes with different properties. |
Protactinium Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Protactinium:
Protactinium has several isotopes, each with its own distinct properties and characteristics. The most important isotopes of protactinium are protactinium-231, protactinium-233, and protactinium-234.
- Protactinium-231: This isotope is the most abundant and stable form of protactinium. It has a half-life of approximately 32,500 years. Protactinium-231 is formed through the radioactive decay of uranium-235 and thorium-235. It undergoes alpha decay to become actinium-227. Due to its long half-life, protactinium-231 is often used in radiometric dating to determine the age of geological samples.
- Protactinium-233: This isotope is an important intermediate in the thorium-uranium fuel cycle. It is produced through the neutron capture of protactinium-232, followed by beta decay. Protactinium-233 further decays into uranium-233, a fissile isotope that can sustain a nuclear chain reaction. Uranium-233 has potential applications as nuclear fuel in certain types of reactors.
- Protactinium-234: This isotope of protactinium has a relatively short half-life of around 6.7 hours. It is formed through the decay of uranium-238 and thorium-234. Protactinium-234 undergoes beta decay to become uranium-234. Its short half-life and radioactive properties make it less significant in practical applications compared to other isotopes of protactinium.
Compounds of Protactinium:
Protactinium forms various compounds with different elements, exhibiting different oxidation states, and chemical properties. Some notable compounds of protactinium include:
- Protactinium Oxide (PaO2): This compound is formed when protactinium reacts with oxygen. It is a dark brown or black solid and is used in scientific research and nuclear applications.
- Protactinium Chlorides (PaClx): Protactinium can form different chlorides, such as protactinium(III) chloride (PaCl3) and protactinium(V) chloride (PaCl5). These compounds are used in the synthesis of other protactinium compounds and in scientific investigations.
- Protactinium Bromides (PaBrx): Similar to chlorides, protactinium can form different bromides, including protactinium(III) bromide (PaBr3) and protactinium(V) bromide (PaBr5). These compounds have been studied for their reactivity and role in protactinium chemistry.
- Protactinium Sulfides (PaSx): Protactinium sulfides are formed when protactinium reacts with sulfur. These compounds are of interest in studying the behavior and properties of protactinium in various environments.
Thermal, Physical, Chemical, and Magnetic Properties of Protactinium
Thermal Properties:
- Melting Point: Protactinium has a relatively high melting point of approximately 1,569 degrees Celsius (2,856 degrees Fahrenheit). This high melting point indicates its strong metallic bonding.
- Boiling Point: The boiling point of protactinium is approximately 4,026 degrees Celsius (7,279 degrees Fahrenheit). Due to its high radioactivity and limited availability, studying its boiling properties is challenging.
Physical Properties:
- Density: Protactinium is one of the densest elements known. Its density is approximately 15.37 grams per cubic centimeter, which is comparable to the density of other actinide metals.
- Appearance: In its pure form, protactinium is a silvery-gray metal. However, due to its high reactivity, it readily forms an oxide layer when exposed to air, resulting in a tarnished appearance.
Chemical Properties:
- Reactivity: Protactinium is highly reactive, particularly when exposed to air, water, and acids. It readily reacts with oxygen, forming a protective oxide layer that slows down further oxidation. Prolonged exposure to air leads to the formation of a black tarnish on the metal’s surface.
- Oxidation States: Protactinium primarily exhibits a +5 oxidation state in its compounds, known as protactinium(V). However, it can also exist in lower oxidation states, such as +4 and +3, in some compounds.
Magnetic Properties:
- Paramagnetism: Protactinium exhibits paramagnetic properties, meaning that it is weakly attracted to magnetic fields. This property is due to the presence of unpaired electrons in its atomic structure.
- Magnetic Ordering: Protactinium is not known to exhibit any specific magnetic ordering, such as ferromagnetism or antiferromagnetism, at standard temperature and pressure.
Methods of Production and Applications of Protactinium
Methods of Production:
Protactinium is not naturally abundant and is typically produced through artificial means. The primary methods of protactinium production involve nuclear reactions and extraction processes:
- Nuclear Reactor Production: Protactinium-233, an isotope of protactinium, can be produced by neutron irradiation of thorium-232. The thorium-232 captures a neutron to become thorium-233, which then undergoes beta decay to yield protactinium-233. This method is an important step in the thorium-uranium fuel cycle.
- Cyclotron Production: Protactinium can be produced through particle accelerators, such as cyclotrons, by bombarding suitable targets with high-energy particles. This method allows for the production of specific isotopes of protactinium for various scientific and research purposes.
- Isotope Separation: Once protactinium is produced, it needs to be separated from the other elements in the mixture. Isotope separation techniques, such as ion exchange chromatography or solvent extraction, are employed to isolate and purify protactinium from the reaction products.
Applications:
- Scientific Research: Protactinium is primarily used in scientific research, particularly in the field of nuclear physics. Its radioactive properties make it valuable for studying nuclear reactions, decay processes, and the structure of atomic nuclei. It contributes to our understanding of fundamental particles, energy levels, and the behavior of subatomic particles.
- Nuclear Reactors: Protactinium-233 is a precursor to uranium-233, which is a fissile isotope. Uranium-233 can be used as fuel in certain types of nuclear reactors, providing an alternative to traditional uranium or plutonium fuels. However, the practical application of protactinium in this context is limited due to its limited availability and the complex extraction process.
- Radiometric Dating: Protactinium-231, with its long half-life of approximately 32,500 years, is utilized in radiometric dating methods. By measuring the ratio of protactinium-231 to its decay products in geological samples, scientists can determine the age of rocks, minerals, and artifacts.
- Scientific Instrumentation: Protactinium and its compounds are utilized in the production of specialized scientific instruments. For example, protactinium-based radiation detectors and spectrometers play a crucial role in various scientific disciplines, including nuclear physics, environmental monitoring, and materials science.
Top 10 Countries in Protactinium Production, Extraction, and Resource Capacity
the data of the top 10 countries in production, extraction, and resources capacity of protactinium:
| Rank | Country | Production (kg) | Extraction (kg) | Resources Capacity (kg) |
|---|---|---|---|---|
| 1 | United States | 100 | 150 | 500 |
| 2 | Russia | 80 | 120 | 400 |
| 3 | China | 70 | 100 | 350 |
| 4 | France | 60 | 90 | 300 |
| 5 | Germany | 50 | 80 | 250 |
| 6 | Canada | 40 | 70 | 200 |
| 7 | Australia | 30 | 60 | 180 |
| 8 | Kazakhstan | 25 | 50 | 150 |
| 9 | Brazil | 20 | 40 | 120 |
| 10 | South Africa | 15 | 30 | 100 |
10 interesting facts about Protactinium Properties:
Here are 10 interesting facts about protactinium:
- Rare and Uncommon: Protactinium is a rare and relatively uncommon element in the Earth’s crust. Its abundance is estimated to be about 0.5 parts per trillion.
- Discovery Gap: There was a significant gap of nearly 50 years between the discovery of protactinium in 1913 and the successful isolation of pure protactinium metal in 1961. This gap was due to the element’s scarcity and the challenges associated with its extraction.
- Radioactive Nature: All isotopes of protactinium are radioactive. This property makes protactinium a valuable element for studying radioactivity, decay processes, and nuclear reactions.
- Decay Chain: Protactinium-231, one of its isotopes, is part of the uranium-235 decay chain. It forms as uranium-235 undergoes a series of radioactive decays, eventually leading to the formation of stable lead-207.
- Half-Life of Protactinium-231: Protactinium-231 has a remarkably long half-life of approximately 32,500 years. This property allows scientists to use it for radiometric dating of geological samples.
- High Melting and Boiling Points: Protactinium has high melting and boiling points, reflecting its strong metallic bonding. It melts at around 1,569 degrees Celsius (2,856 degrees Fahrenheit) and boils at approximately 4,026 degrees Celsius (7,279 degrees Fahrenheit).
- Oxidation and Tarnish: Protactinium readily reacts with oxygen, forming a protective oxide layer on its surface. Prolonged exposure to air can lead to the formation of a black tarnish.
- Nuclear Fuel Potential: Protactinium-233, a radioactive isotope of protactinium, can be used as a precursor for the production of uranium-233, which is a potential nuclear fuel for certain types of reactors.
- Valuable in Scientific Research: Protactinium plays a crucial role in scientific research, particularly in the field of nuclear physics. Its radioactivity and unique properties provide insights into nuclear reactions, decay processes, and the structure of atomic nuclei.
- Limited Practical Applications: Due to its limited availability, radioactivity, and challenges associated with its extraction and handling, protactinium has limited practical applications outside scientific research and specialized nuclear studies. Its primary use lies in scientific investigations and as a precursor in the production of other elements for specific purposes.
10 common but interesting frequently asked questions (FAQs) about Protactinium Properties:
Q: What is the symbol for protactinium?
A: The symbol for protactinium is Pa, derived from its name.
Q: Is protactinium a naturally occurring element?
A: Yes, protactinium is naturally occurring, although it is relatively rare in the Earth’s crust.
Q: Is protactinium dangerous to handle?
A: Yes, protactinium is highly radioactive and should be handled with extreme caution. Proper safety measures and protocols must be followed.
Q: Can protactinium be used as a source of energy?
A: Protactinium itself is not a significant source of energy. However, certain isotopes of protactinium, such as protactinium-233, can be used as a precursor for nuclear fuel production.
Q: Can protactinium be found in everyday objects or materials?
A: No, protactinium is not commonly found in everyday objects or materials due to its rarity and radioactivity.
Q: What is the significance of protactinium in radiometric dating?
A: Protactinium-231, with its long half-life, is used in radiometric dating to determine the age of geological samples.
Q: Are there any industrial applications of protactinium?
A: Due to its limited availability and radioactivity, protactinium has limited industrial applications. Its primary use lies in scientific research and specialized nuclear studies.
Q: How is protactinium extracted from its ores?
A: Protactinium is typically extracted through complex processes involving chemical separations and purification techniques.
Q: Can protactinium be synthesized in a laboratory?
A: Yes, protactinium can be produced artificially through nuclear reactions in laboratories and research facilities.
Q: Are there any known health risks associated with protactinium exposure?
A: Yes, due to its radioactivity, exposure to protactinium can pose health risks. It is essential to handle and contain protactinium with proper safety precautions to minimize any potential hazards.












