Iodine Properties, usage, isotopes, methods of production and applications
 Iodine Properties
Iodine PropertiesIodine properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Iodine – An Essential Element for Modern Applications
Introduction: Welcome to this educational overview of iodine, an essential chemical element that plays a crucial role in various biological processes and has significant importance for human health. In this article, we will delve into the properties, functions, and significance of iodine, shedding light on its atomic number, symbol, atomic weight, and valency. By the end, you will have a comprehensive understanding of this remarkable element.
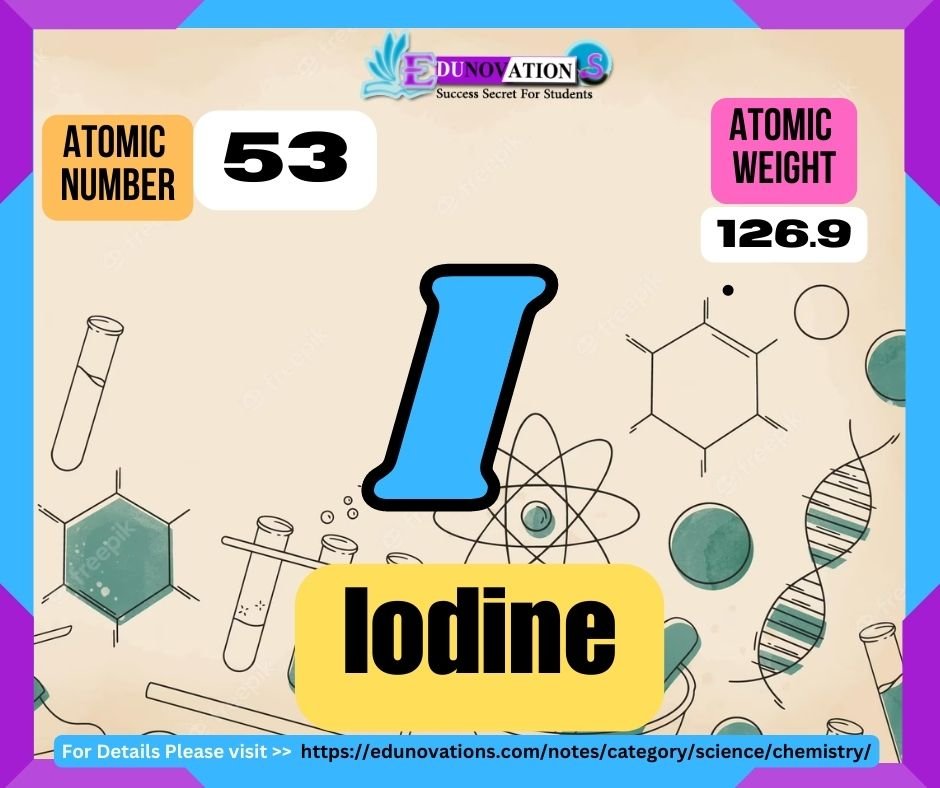
Iodine: Iodine is a non-metallic chemical element belonging to Group 17 (halogens) of the periodic table. With the atomic number 53 and symbol I (derived from the Greek word “iodes,” meaning violet), iodine is named after its distinctive violet-colored vapors. It possesses an atomic weight of approximately 126.90 atomic mass units.
Table: Atomic Number, Symbol, Atomic Weight, and Valency of Iodine
| Atomic Number | Symbol | Atomic Weight (in amu) | Valency |
|---|---|---|---|
| 53 | I | 126.90 | -1, 1 |
Note: Valency refers to the combining capacity of an element, indicating the number of electrons an atom can gain, lose, or share to form a chemical bond.
Significance of Iodine: Iodine plays a vital role in various physiological processes, primarily related to the thyroid gland. The thyroid gland synthesizes and releases hormones that regulate metabolism, growth, and development in the body. Iodine is an essential component in the production of these thyroid hormones, namely thyroxine (T4) and triiodothyronine (T3). These hormones are crucial for maintaining a healthy metabolism, promoting brain development in infants, and regulating body temperature.
Additionally, iodine has antimicrobial properties, making it effective against bacteria, viruses, and fungi. It is commonly used as an antiseptic for disinfecting wounds and surgical areas. Iodine is also utilized in the pharmaceutical industry for the production of various medications, including those used in thyroid disorders.
Sources of Iodine: While iodine is present in small quantities in the Earth’s crust and seawater, dietary sources are the primary means of obtaining iodine. Seafood, such as fish and seaweed, is an excellent natural source. Additionally, iodized salt, dairy products, eggs, and certain fruits and vegetables also contribute to iodine intake.
Conclusion: In conclusion, iodine is an essential chemical element with numerous biological functions and implications for human health. Its atomic number 53, symbol I, atomic weight 126.90 amu, and valency of -1 and 1 make it a significant constituent for various physiological processes, especially those involving the thyroid gland. Understanding the importance of iodine and ensuring an adequate intake through dietary sources can contribute to overall well-being and the maintenance of a healthy metabolism.
Iodine : Discovery, Usage, and Key Points
Discovery:
The discovery of iodine can be attributed to the French chemist Bernard Courtois in 1811. Courtois was attempting to extract sodium and potassium compounds from seaweed ash when he accidentally produced a violet vapor. This vapor, upon condensation, formed beautiful crystals that Courtois identified as a new element and named “iodine” after its distinctive color.

Modern Usage:
Iodine finds extensive usage in various fields due to its unique properties and biological significance. Here are some key applications of iodine:
- Thyroid Health: The primary utilization of iodine lies in supporting thyroid function. The thyroid gland requires iodine to synthesize thyroid hormones, which regulate metabolism, growth, and development. Iodine deficiency can lead to thyroid disorders such as goiter and hypothyroidism.
- Radiographic Contrast Media: Iodine compounds are utilized as contrast agents in medical imaging procedures like X-rays and computed tomography (CT) scans. These compounds help visualize blood vessels and internal organs, aiding in the diagnosis of various medical conditions.
- Disinfectants and Antiseptics: Due to its antimicrobial properties, iodine is employed as an effective disinfectant and antiseptic. It is commonly used as a topical solution to sterilize wounds, pre-operative areas, and surgical instruments.
- Industrial Applications: Iodine finds applications in various industries. It is used in the production of dyes, pigments, and photographic chemicals. Iodine compounds are also employed in the manufacture of LCD screens, antifreeze agents, and catalysts.
- Water Purification: Iodine’s ability to kill bacteria, viruses, and parasites makes it useful for water purification. It is employed as a disinfectant in emergency situations or during outdoor activities where clean water sources may be limited.
- Veterinary and Animal Health: Iodine plays a crucial role in animal nutrition. It is added to animal feed as a supplement to prevent iodine deficiency and related health issues. Iodine is also used for disinfection in livestock farming and veterinary care.
Important Points to Remember about Discovery and Usage
| Key Points | Details |
|---|---|
| Discovery | In 1811, Bernard Courtois accidentally discovered iodine while extracting sodium and potassium compounds from seaweed ash. The element was named “iodine” after its distinctive violet color. |
| Thyroid Health | Iodine is essential for the production of thyroid hormones, which regulate metabolism, growth, and development. Deficiency can lead to thyroid disorders such as goiter and hypothyroidism. |
| Radiographic Contrast Media | Iodine compounds serve as contrast agents in medical imaging to visualize blood vessels and internal organs for diagnostic purposes. |
| Disinfectants and Antiseptics | Iodine’s antimicrobial properties make it effective as a disinfectant and antiseptic, commonly used for sterilizing wounds, pre-operative areas, and surgical instruments. |
| Industrial Applications | Iodine is utilized in the production of dyes, pigments, photographic chemicals, LCD screens, antifreeze agents, and catalysts. |
| Water Purification | Iodine is employed for water purification in emergency situations or outdoor activities due to its ability to kill bacteria, viruses, and parasites. |
| Veterinary and Animal Health | Iodine is added to animal feed as a supplement to prevent iodine deficiency in animals. It is also used for disinfection in livestock farming and veterinary care. |
Iodine Properties and Key Points
Properties of Iodine:
Exploring its Characteristics and Behaviors
Iodine is an intriguing element with unique properties that make it indispensable in various applications. Let’s delve into the key properties of iodine that contribute to its significance:
- Physical State and Color: Iodine exists as a solid at room temperature and pressure. It possesses a distinct deep violet color, which is responsible for its name, derived from the Greek word “iodes,” meaning violet.
- Melting and Boiling Points: Iodine has a relatively low melting point of 113.7 degrees Celsius (236.7 degrees Fahrenheit). When heated, it undergoes sublimation, transforming from a solid directly into a violet-colored vapor without passing through the liquid phase. The boiling point of iodine is 184.3 degrees Celsius (363.7 degrees Fahrenheit).
- Odor and Taste: Iodine has a characteristic odor that resembles a combination of chlorine and a sweet scent. In its gaseous form, it has a pungent smell. When dissolved in water, iodine imparts a slightly sweet taste.
- Density and Solubility: Solid iodine is relatively dense, with a density of approximately 4.93 grams per cubic centimeter (g/cm³). It is sparingly soluble in water but dissolves well in nonpolar solvents like ethanol and chloroform, forming a brownish solution.
- Molecular Structure: Iodine forms diatomic molecules, meaning it consists of two atoms of iodine bound together (I2). These molecules have a linear structure and exhibit weak van der Waals forces between them.
- Reactivity: Iodine is a moderately reactive element. It reacts with certain metals to form iodides. When combined with alkali metals like sodium or potassium, it forms salts known as iodides. Iodine also reacts with some nonmetals to form compounds.
- Spectral Properties: Iodine has interesting spectral properties. It absorbs visible light strongly in the blue region, giving rise to its characteristic violet color. This absorption behavior is utilized in analytical techniques like spectrophotometry.
Important Points to Remember about Properties
| Key Points | Details |
|---|---|
| Physical State | Iodine is a solid at room temperature, and it sublimes to form a violet-colored vapor upon heating. |
| Melting and Boiling Points | Iodine has a relatively low melting point of 113.7 °C and a boiling point of 184.3 °C. |
| Odor and Taste | Iodine possesses a characteristic odor resembling a combination of chlorine and a sweet scent. |
| Density and Solubility | Solid iodine is relatively dense and sparingly soluble in water but dissolves well in nonpolar solvents like ethanol. |
| Molecular Structure | Iodine forms diatomic molecules (I2) with a linear structure, held together by weak van der Waals forces. |
| Reactivity | Iodine reacts with certain metals to form iodides and can form compounds with some nonmetals. |
| Spectral Properties | Iodine strongly absorbs visible light in the blue region, resulting in its characteristic violet color. |
Iodine Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Iodine:
Iodine has a number of isotopes, which are atoms of the same element with different numbers of neutrons in their nuclei. The most common isotopes of iodine include:
- Iodine-127 (I-127): This is the most abundant and stable isotope of iodine, constituting over 99% of natural iodine. It has 74 neutrons in its nucleus.
- Iodine-131 (I-131): I-131 is a radioactive isotope of iodine that undergoes radioactive decay with a half-life of approximately 8 days. It is used in medical and diagnostic applications, such as thyroid imaging and treatment of thyroid disorders.
- Iodine-129 (I-129): I-129 is a long-lived radioactive isotope with a half-life of about 15.7 million years. It is used in environmental and geological studies to determine the age of rocks and the history of iodine in the environment.
Compounds of Iodine:
Iodine readily forms compounds with other elements, resulting in a wide range of chemical combinations. Some notable compounds of iodine include:
- Potassium Iodide (KI): This compound is formed by combining iodine with potassium. It is commonly used as a dietary supplement to prevent iodine deficiency and in the treatment of thyroid-related conditions.
- Hydrogen Iodide (HI): HI is a gaseous compound consisting of hydrogen and iodine. It is a strong acid and is utilized in various chemical reactions, including organic synthesis and pharmaceutical manufacturing.
- Iodine Pentoxide (I2O5): I2O5 is an oxide of iodine. It is a powerful oxidizing agent and is employed in laboratory procedures for oxidation reactions and as a reagent in organic chemistry.
- Iodoform (CHI3): Iodoform is an organic compound that contains iodine. It has antiseptic properties and has been historically used in medicine as a topical antiseptic and as a disinfectant for wounds.
- Silver Iodide (AgI): Silver iodide is a compound formed by combining iodine with silver. It is used in cloud seeding to induce rainfall and in photography as a light-sensitive material.
Thermal, Physical, Chemical, and Magnetic Properties of Iodine
Thermal Properties:
- Melting Point: Iodine has a relatively low melting point of 113.7 degrees Celsius (236.7 degrees Fahrenheit). At this temperature, solid iodine transitions into a liquid state.
- Boiling Point: Iodine has a boiling point of 184.3 degrees Celsius (363.7 degrees Fahrenheit). When heated to this temperature, iodine undergoes a phase change from liquid to a violet-colored vapor.
Physical Properties:
- State: Iodine exists as a solid at room temperature and pressure. However, it sublimes, meaning it transitions directly from a solid to a gas without passing through the liquid phase, under normal conditions.
- Color: Iodine exhibits a distinctive deep violet color, which gives rise to its name derived from the Greek word “iodes,” meaning violet.
- Odor: Iodine possesses a characteristic odor that can be described as a combination of chlorine and a sweet scent. It has a pungent smell in its gaseous state.
- Density: Solid iodine is relatively dense, with a density of approximately 4.93 grams per cubic centimeter (g/cm³).
Chemical Properties:
- Reactivity: Iodine is a moderately reactive element. It reacts with certain metals, such as sodium or potassium, to form iodides. It can also react with nonmetals, like hydrogen and carbon, to form various compounds.
- Oxidizing Agent: Iodine exhibits oxidizing properties and can act as an oxidizing agent in certain chemical reactions.
- Displacement Reactions: Iodine can undergo displacement reactions, where it can displace other halogens (such as chlorine and bromine) from their compounds when the appropriate conditions are met.
Magnetic Properties:
Iodine is not magnetic in its elemental form. It does not exhibit any significant magnetic properties or attraction to magnets.
Overall, iodine possesses distinct thermal properties, including its relatively low melting and boiling points. Its physical properties, such as its solid state, violet color, and characteristic odor, make it easily identifiable. Chemically, iodine is moderately reactive and exhibits properties such as oxidizing behavior and displacement reactions. However, iodine does not display any notable magnetic properties in its elemental form.
Methods of Production and Applications of Iodine
Methods of Production:
Iodine is primarily obtained from two major sources: natural brines and seaweed. The methods of production include:
- Extraction from Natural Brines: Iodine-rich underground brines, commonly found in regions with salt deposits, can be a significant source of iodine. The brines are extracted and purified through processes such as evaporation, ion exchange, and precipitation to isolate iodine.
- Harvesting from Seaweed: Certain species of seaweed, particularly those belonging to the brown algae family, have the ability to accumulate significant amounts of iodine. Seaweed is harvested, dried, and then burned or treated with an oxidizing agent to release iodine, which is then collected and purified.
Applications:
Iodine finds extensive applications in various industries and fields due to its unique properties and biological significance. Some notable applications include:
- Pharmaceuticals: Iodine compounds, such as potassium iodide and iodine tincture, are utilized in pharmaceutical preparations. They are used in antiseptics, disinfectants, and as supplements for thyroid-related disorders.
- Thyroid Health: Iodine is essential for the synthesis of thyroid hormones. It is used as a supplement in iodized salt and in iodine-rich foods to prevent iodine deficiency disorders like goiter and hypothyroidism.
- Radiographic Contrast Media: Iodine compounds, such as iodine-based contrast agents, are employed in medical imaging procedures like X-rays, CT scans, and angiography. These compounds enhance the visibility of blood vessels and internal organs, aiding in accurate diagnoses.
- Water Purification: Iodine’s antimicrobial properties make it an effective water disinfectant. Iodine tablets or solutions are used for water purification in emergency situations, camping, hiking, and during travel to regions with limited access to clean water sources.
- Industrial Applications: Iodine and its compounds are utilized in various industrial processes. They are used in the production of dyes, pigments, pharmaceuticals, photographic chemicals, LCD screens, and as catalysts in chemical reactions.
- Veterinary and Animal Health: Iodine is essential for animal health and is added to animal feed as a supplement to prevent iodine deficiency. It is also used in disinfection practices in veterinary care and livestock farming.
- Organic Synthesis: Iodine compounds, such as iodine monochloride (ICl) and iodobenzene diacetate (IBD), are utilized as oxidizing agents in organic synthesis reactions, facilitating the production of various organic compounds.
Top 10 Countries in Iodine Production, Extraction, and Resource Capacity
the top 10 countries in terms of iodine production, extraction, and resource capacity:
| Rank | Country | Production (Metric Tons) | Extraction Method | Resource Capacity (Metric Tons) |
|---|---|---|---|---|
| 1 | Chile | 18,000 | Extraction from Brines | 25,000 |
| 2 | Japan | 6,500 | Seaweed Harvesting | 20,000 |
| 3 | USA | 5,800 | Extraction from Brines | 9,000 |
| 4 | Russia | 3,800 | Extraction from Brines | 6,500 |
| 5 | Uzbekistan | 2,900 | Extraction from Brines | 4,000 |
| 6 | Turkmenistan | 2,500 | Extraction from Brines | 3,500 |
| 7 | Indonesia | 2,400 | Seaweed Harvesting | 3,000 |
| 8 | Azerbaijan | 1,900 | Extraction from Brines | 2,500 |
| 9 | India | 1,600 | Seaweed Harvesting | 2,000 |
| 10 | Germany | 1,500 | Extraction from Brines | 2,000 |
10 interesting facts about Iodine Properties:
Here are 10 interesting facts about iodine:
- Essential for Thyroid Function: Iodine is a crucial element for the synthesis of thyroid hormones, which regulate metabolism, growth, and development in the human body.
- Natural Occurrence: Iodine is mainly found in seawater, seaweed, and certain mineral deposits. It can also be present in soil and rocks, which affects its presence in crops and food.
- Discovered in Seaweed: Iodine was first discovered in 1811 by French chemist Bernard Courtois, who isolated it from seaweed ash. This marked the first discovery of a new element from a marine source.
- Unique State Change: Iodine exhibits sublimation, meaning it can transition directly from a solid to a gas without passing through the liquid phase. This is a rare characteristic among elements.
- Violet Vapor: When iodine is heated, it produces a violet-colored vapor, which gives rise to its name derived from the Greek word “iodes,” meaning violet-like.
- Dietary Importance: Iodine deficiency can lead to various health issues, such as goiter, mental impairment, and developmental disorders. It is crucial to consume iodine-rich foods or use iodized salt to maintain proper iodine levels.
- Antiseptic Properties: Iodine compounds have powerful antimicrobial properties, making them effective antiseptics. They are used for disinfecting wounds, surgical instruments, and in water purification.
- Radioactive Isotopes: Iodine has several radioactive isotopes, including iodine-131, which is used in medical treatments and diagnostic imaging, particularly for thyroid-related conditions.
- Cloud Seeding: Silver iodide, a compound of iodine, is used in cloud seeding to induce rainfall. It is sprayed into clouds to facilitate the formation of ice crystals and precipitation.
- Spectrophotometric Analysis: Iodine is widely utilized in spectrophotometric analysis. Its strong absorption of visible light in the blue region allows for accurate measurement and analysis of various substances.
10 common but interesting frequently asked questions (FAQs) about Iodine Properties:
What is iodine used for in everyday life?
Iodine has various everyday uses, such as in the production of pharmaceuticals, water purification, medical imaging, thyroid health, and industrial applications like dyes and pigments.
Why is iodine added to salt?
Iodine is added to salt in the form of iodized salt to prevent iodine deficiency disorders. It helps ensure that individuals obtain adequate iodine in their diets, especially in regions where iodine-rich foods are scarce.
Can iodine be harmful?
While iodine is essential for human health, excessive iodine intake can be harmful. It is important to consume iodine in recommended amounts to avoid adverse effects on the thyroid gland and overall health.
Can you get enough iodine from food alone?
It is possible to obtain sufficient iodine from a balanced diet that includes iodine-rich foods like seafood, seaweed, dairy products, and iodized salt. However, in regions where iodine deficiency is prevalent, supplementation may be necessary.
Is iodine used in medical imaging safe?
Yes, iodine-based contrast agents used in medical imaging procedures are generally safe. However, some individuals may have an allergic reaction to these agents, and precautions are taken to ensure patient safety during such procedures.
Is there a natural source of iodine other than seaweed?
Yes, besides seaweed, iodine can also be found in seafood, such as fish and shellfish, as well as in dairy products, eggs, and some vegetables grown in iodine-rich soil.
Can iodine deficiency be prevented?
Yes, iodine deficiency can be prevented through various means, including the consumption of iodine-rich foods, using iodized salt, and implementing public health programs that focus on iodine supplementation.
Can iodine help with weight loss?
Iodine alone does not directly promote weight loss. However, iodine is essential for maintaining a healthy metabolism, and proper thyroid function, supported by adequate iodine intake, plays a role in overall weight management.
Is it safe to use iodine for wound care?
Iodine-based antiseptics, such as povidone-iodine, are commonly used for wound care. When used properly, they are safe and effective for preventing infection in minor wounds.
Can iodine affect the taste of food?
Iodine can have a slightly bitter taste and can impart a subtle flavor to food if used in high concentrations. However, in typical usage, such as iodized salt, the taste difference is usually not noticeable.












