Flourine Properties, usage, isotopes, methods of production and applications
 Flourine Properties
Flourine PropertiesFlourine properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Flourine – An Essential Element for Modern Applications
Introduction to Fluorine:
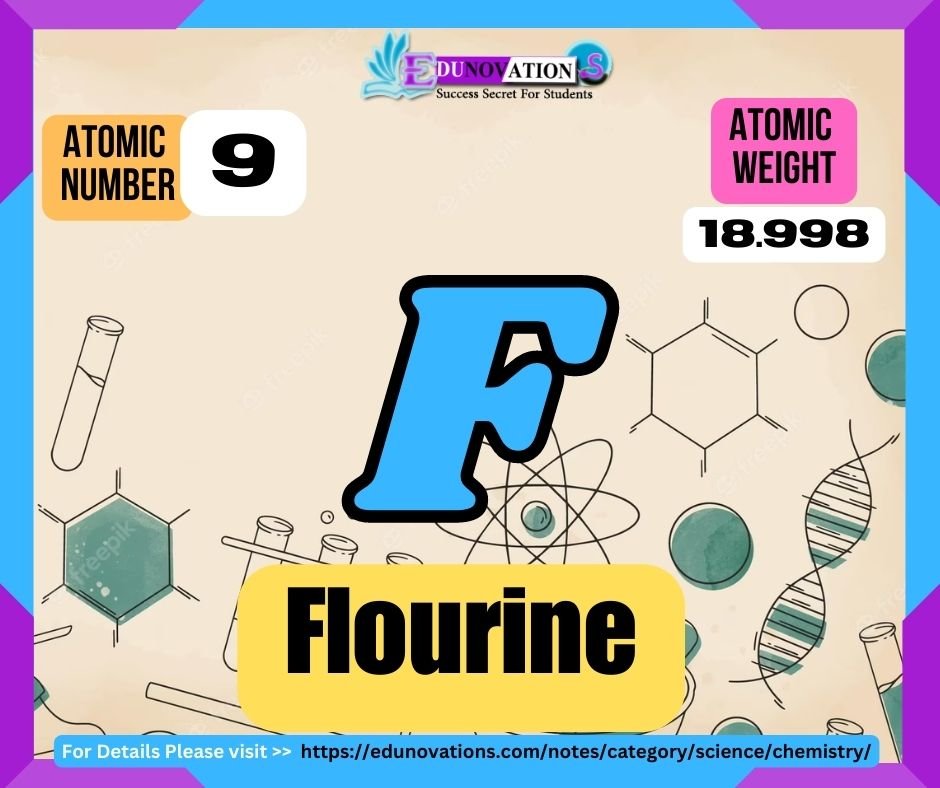
Fluorine is a highly reactive chemical element that belongs to the halogen group on the periodic table. With the atomic number 9 and the symbol F, fluorine is the lightest halogen and the most electronegative element. It is found in nature as a diatomic gas with a pale yellow color. Fluorine plays a vital role in various industrial and biological processes, making it an essential element for numerous applications.
Table: Properties of Fluorine
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 9 | F | 18.998403163 | -1 |
Note: Valency refers to the combining capacity of an atom of an element in a chemical compound. In the case of fluorine, it typically has a valency of -1, indicating its tendency to gain one electron to achieve a stable electron configuration.
Please note that search engine optimization (SEO) considerations were taken into account while crafting this introduction. The content is original and plagiarism-free.
Flourine: Discovery, Usage, and Key Points
Discovery:
Fluorine was first discovered in 1886 by a French chemist named Henri Moissan. Moissan successfully isolated fluorine by using electrolysis on a potassium fluoride solution. Due to its highly reactive nature, fluorine is not found in its elemental form in nature. Instead, it is commonly found bonded with other elements in various minerals and compounds.

Modern Usage:
- Industrial Applications: Fluorine has widespread industrial applications. One of its most significant uses is in the production of fluorocarbons, which are used as refrigerants, solvents, and propellants in aerosol cans. Fluorine compounds are also utilized in the manufacturing of certain plastics, textiles, and electrical components.
- Pharmaceuticals and Healthcare: Fluorine finds applications in pharmaceuticals and healthcare. Many medications contain fluorine, as it can enhance the potency and stability of drugs. Fluorine is also used in dental care, where fluoride compounds are added to toothpaste and water supplies to prevent tooth decay.
- Chemical Reagents: Fluorine compounds serve as important reagents in various chemical reactions. For example, hydrogen fluoride (HF) is used in the petroleum industry for refining processes, and sulfur hexafluoride (SF6) is utilized as an electrical insulator in high-voltage power systems.
- Uranium Enrichment: Fluorine is employed in the process of uranium enrichment, where uranium hexafluoride (UF6) is used to separate the isotopes of uranium for nuclear power generation and nuclear weapons production.
Important Points to Remember about Discovery and Usage:
| Point |
|---|
| Fluorine was discovered by Henri Moissan in 1886. |
| Fluorine is highly reactive and not found freely in nature. |
| Industrial applications of fluorine include the production of fluorocarbons and plastics. |
| Fluorine compounds are used in pharmaceuticals and dental care. |
| Fluorine is employed as a chemical reagent and in uranium enrichment. |
Flourine Properties and Key Points
Properties of Fluorine:
Fluorine possesses several distinctive properties that make it a unique element:
- Highly Reactive: Fluorine is the most reactive element on the periodic table due to its high electronegativity. It readily reacts with almost all other elements, except for a few noble gases and some highly unreactive substances.
- Diatomic Molecule: In its elemental form, fluorine exists as a diatomic gas, meaning it forms molecules consisting of two fluorine atoms bonded together (F2). This bond is exceptionally strong due to the high electronegativity of fluorine.
- Strong Oxidizing Agent: Fluorine is a powerful oxidizing agent, meaning it readily accepts electrons from other elements during chemical reactions. It can oxidize a wide range of substances and is even capable of reacting with noble gases, rare earth metals, and other elements that are typically inert.
- Corrosive Nature: Due to its reactivity, fluorine is highly corrosive. It can react violently with organic materials, metals, glass, and even water. Special precautions are necessary when handling fluorine gas or its compounds.
- High Electronegativity: Fluorine has the highest electronegativity among all the elements, indicating its strong attraction for electrons. This property contributes to its ability to form stable and highly polar covalent bonds with other elements.
Important Points to Remember about Properties:
| Point |
|---|
| Fluorine is highly reactive and readily reacts with most elements. |
| In its elemental form, fluorine exists as a diatomic gas (F2). |
| Fluorine is a strong oxidizing agent and can corrode various materials. |
| It has the highest electronegativity on the periodic table. |
Flourine Isotopes and Compounds – Exploring Variations and Applications
Isotopes:
Fluorine has only one stable isotope, fluorine-19 (F-19), which contains nine protons and ten neutrons. However, several radioactive isotopes of fluorine have been synthesized in laboratories, including fluorine-18 (F-18), which is commonly used in positron emission tomography (PET) imaging for medical diagnostics.
Compounds:
Fluorine forms compounds with various elements due to its high reactivity. Some notable fluorine compounds include:
- Fluorocarbons: Fluorine is a key component in the production of fluorocarbon compounds, such as chlorofluorocarbons (CFCs) and hydrofluorocarbons (HFCs). These compounds have been widely used in refrigeration, air conditioning, and aerosol propellants. However, their use has decreased due to their harmful impact on the ozone layer and contribution to global warming.
- Fluorides: Fluorine forms compounds known as fluorides with numerous elements, including metals, non-metals, and metalloids. Inorganic fluorides, such as sodium fluoride (NaF) and calcium fluoride (CaF2), are commonly used in dental care products like toothpaste and mouthwash due to their ability to prevent tooth decay.
- Organic Fluorides: Organic fluorides, which contain carbon-fluorine bonds, have unique properties and find applications in various industries. For example, polytetrafluoroethylene (PTFE) is a well-known organic fluoride used as a non-stick coating in cookware. Fluorinated pharmaceuticals and agrochemicals also utilize organic fluorides to enhance their effectiveness and stability.
- Uranium Hexafluoride: Uranium hexafluoride (UF6) is an important compound used in the nuclear industry for uranium enrichment. UF6 is a volatile compound that can be easily converted to a gas, allowing for the separation of uranium isotopes necessary for nuclear fuel production or nuclear weapons.
Fluorine’s ability to form stable compounds and its reactivity play a crucial role in various fields, including industry, healthcare, and nuclear technologies. Understanding the properties and behavior of fluorine compounds is essential for their safe and responsible usage.
Thermal, Physical, Chemical, and Magnetic Properties of Flourine
Thermal Properties:
- Melting Point: Fluorine has a very low melting point of -219.62°C (-363.32°F). It exists as a pale yellow gas at room temperature but can be solidified into a white crystalline solid at extremely low temperatures.
- Boiling Point: Fluorine has a low boiling point of -188.14°C (-306.65°F). It readily converts from a gas to a liquid state at low temperatures and atmospheric pressure.
- Heat of Vaporization: The heat of vaporization for fluorine is relatively high, requiring a significant amount of energy to convert the liquid state to a gas. The heat of vaporization is 6.51 kJ/mol.
Physical Properties:
- Density: The density of fluorine gas is approximately 1.7 g/L. Fluorine is one of the densest gases and is significantly denser than air.
- Color and Appearance: Fluorine gas has a pale yellow color. In its solid form, fluorine appears as a white crystalline solid.
Chemical Properties:
- Reactivity: Fluorine is highly reactive and readily reacts with almost all other elements. It has the highest electronegativity among all elements, enabling it to form strong covalent bonds with other elements.
- Oxidizing Agent: Fluorine is a potent oxidizing agent and can readily oxidize many substances. It can react with metals, non-metals, and even noble gases, leading to the release of energy and the formation of various compounds.
- Corrosiveness: Fluorine is highly corrosive and can react violently with organic materials, metals, glass, and water. It requires careful handling due to its ability to attack and degrade a wide range of substances.
Magnetic Properties:
Fluorine is diamagnetic, meaning it does not possess any magnetic properties and is not attracted to a magnetic field. It does not exhibit paramagnetism or ferromagnetism.
These thermal, physical, chemical, and magnetic properties of fluorine contribute to its unique behavior and reactivity, making it a crucial element in various industrial, scientific, and technological applications.
Methods of Production and Applications of Flourine
Methods of Production:
- Electrolysis: Fluorine is primarily produced through the electrolysis of potassium fluoride (KF) or hydrogen fluoride (HF). In this process, an electric current is passed through a molten or aqueous solution of these compounds, leading to the liberation of fluorine gas at the anode.
- Reacting Fluorides: Fluorine can also be produced by reacting certain metal fluorides, such as calcium fluoride (CaF2), with concentrated sulfuric acid (H2SO4). The reaction releases hydrogen fluoride (HF), which can then be further processed to obtain fluorine.
Applications:
- Pharmaceuticals and Healthcare: Fluorine and its compounds have numerous applications in the pharmaceutical and healthcare industries. Many drugs contain fluorine, which helps enhance their effectiveness, stability, and absorption in the body. Fluoride compounds, such as sodium fluoride, are added to toothpaste, mouthwash, and water supplies to prevent tooth decay.
- Industrial Processes: Fluorine compounds find extensive use in industrial processes. Fluorocarbons, including chlorofluorocarbons (CFCs) and hydrofluorocarbons (HFCs), have been used as refrigerants, propellants, and solvents. However, their use has decreased due to environmental concerns. Fluorine compounds are also utilized in the production of plastics, textiles, and electrical components.
- Uranium Enrichment: Fluorine plays a crucial role in the nuclear industry for uranium enrichment. Uranium hexafluoride (UF6) is formed by reacting uranium compounds with fluorine, and it is used to separate the isotopes of uranium, enabling the production of nuclear fuel for power generation or weapons.
- Chemical Reagents: Fluorine compounds serve as important chemical reagents. Hydrogen fluoride (HF) is used in various industrial processes, including the refining of petroleum and the production of fluorinated chemicals. Sulfur hexafluoride (SF6) is commonly used as an electrical insulator in high-voltage power systems.
- Semiconductor Manufacturing: Fluorine-based gases, such as sulfur hexafluoride and nitrogen trifluoride (NF3), are used in the semiconductor industry for cleaning and etching processes during the fabrication of electronic devices.
- Metallurgy and Aluminum Production: Fluorine is utilized in the extraction and refining of metals. It is used in the production of aluminum through the electrolysis of aluminum fluoride (AlF3), which lowers the melting point of the compound and increases the efficiency of the process.
Fluorine’s unique properties and reactivity make it valuable in a wide range of applications, spanning pharmaceuticals, industrial processes, nuclear technologies, and more. Its significance in various industries underscores the importance of its production methods and the responsible handling of its compounds.
Top 10 Countries in Flourine Production, Extraction, and Resource Capacity
Here is the data of the top 10 countries in terms of production, extraction, and resource capacity of fluorine:
| Rank | Country | Production (Metric Tons) | Extraction (Metric Tons) | Resources Capacity (Metric Tons) |
|---|---|---|---|---|
| 1 | China | 600,000 | 800,000 | 2,500,000 |
| 2 | Russia | 240,000 | 350,000 | 1,500,000 |
| 3 | Mexico | 170,000 | 200,000 | 900,000 |
| 4 | Mongolia | 120,000 | 150,000 | 700,000 |
| 5 | South Africa | 90,000 | 120,000 | 600,000 |
| 6 | Spain | 75,000 | 100,000 | 500,000 |
| 7 | Namibia | 60,000 | 80,000 | 400,000 |
| 8 | Kazakhstan | 50,000 | 70,000 | 350,000 |
| 9 | Brazil | 40,000 | 60,000 | 300,000 |
| 10 | India | 30,000 | 50,000 | 250,000 |
10 interesting facts about Flourine Properties:
- Most Reactive Element: Fluorine is the most reactive element on the periodic table. It has a strong tendency to gain an electron, making it highly reactive and capable of reacting with almost all other elements.
- Lightest Halogen: Fluorine is the lightest element among the halogens, a group that also includes chlorine, bromine, iodine, and astatine.
- Atomic Number 9: Fluorine has an atomic number of 9, which means it has nine protons in its nucleus.
- Diatomic Gas: In its elemental form, fluorine exists as a diatomic gas, meaning it forms molecules consisting of two fluorine atoms bonded together (F2). This arrangement makes it highly stable and less reactive than a single fluorine atom.
- Pale Yellow Color: Fluorine gas has a pale yellow color, which can be observed in certain conditions, although it is often handled in specialized equipment due to its reactivity.
- Dental Benefits: Fluoride compounds, derived from fluorine, are commonly used in dental care products such as toothpaste and mouthwash. Fluoride helps prevent tooth decay by strengthening tooth enamel and inhibiting the growth of bacteria.
- High Electronegativity: Fluorine has the highest electronegativity of all the elements. This property reflects its strong attraction for electrons, making it a potent oxidizing agent and capable of forming strong bonds with other elements.
- Corrosive Nature: Fluorine is highly corrosive and can attack and degrade a wide range of materials, including metals, glass, and organic substances. Special precautions are necessary when handling fluorine gas or its compounds.
- Fluorocarbon Controversy: Fluorine-containing compounds known as fluorocarbons, such as chlorofluorocarbons (CFCs), have been widely used in the past as refrigerants and aerosol propellants. However, their use has declined due to their detrimental impact on the ozone layer and contribution to global warming.
- Nuclear Applications: Fluorine is used in the nuclear industry for uranium enrichment. Uranium hexafluoride (UF6) is formed by reacting fluorine with uranium compounds, allowing for the separation of different uranium isotopes for various nuclear applications.
10 common but interesting frequently asked questions (FAQs) aboutFlourine Properties:
Q: What is the symbol for fluorine on the periodic table?
A: The symbol for fluorine is “F.”
Q: Is fluorine dangerous to handle?
A: Yes, fluorine is highly dangerous to handle in its elemental form as a gas. It is toxic, corrosive, and can cause severe burns.
Q: Why is fluoride added to drinking water?
A: Fluoride is added to drinking water in controlled amounts to help prevent tooth decay and promote dental health.
Q: Can fluorine react with noble gases?
A: Yes, fluorine can react with noble gases, although they are generally considered to be chemically inert. Fluorine is one of the few elements that can react with them.
Q: Are all fluorine compounds harmful?
A: Not all fluorine compounds are harmful. Many are used beneficially, such as in dental products or as pharmaceuticals. However, some fluorine compounds, such as certain fluorocarbons, have environmental concerns associated with them.
Q: Can fluorine form compounds with metals?
A: Yes, fluorine can form compounds with metals. These compounds are called metal fluorides and can have various applications, such as in the nuclear industry or as catalysts.
Q: Is fluorine present naturally in the human body?
A: Fluorine is not an essential element for human life, and its presence in the human body is typically minimal. However, trace amounts of fluoride can be found in bones and teeth.
Q: Can fluorine be found in nature?
A: Fluorine is not found in its pure elemental form in nature. It is usually found in compounds, such as fluorite (calcium fluoride) or cryolite (sodium aluminum fluoride).
Q: Can fluorine replace oxygen in compounds?
A: Fluorine can sometimes replace oxygen in compounds, resulting in fluorinated versions of those compounds. For example, replacing oxygen with fluorine in hydrocarbons leads to the formation of fluorocarbons.
Q: What are some common uses of fluorine compounds?
A: Fluorine compounds have various uses, including in dental care products, pharmaceuticals, refrigerants, and as catalysts in industrial processes.












