Erbium Properties, usage, isotopes, methods of production and applications
 Erbium Properties
Erbium PropertiesErbium properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Erbium – An Essential Element for Modern Applications
Introduction to Erbium:
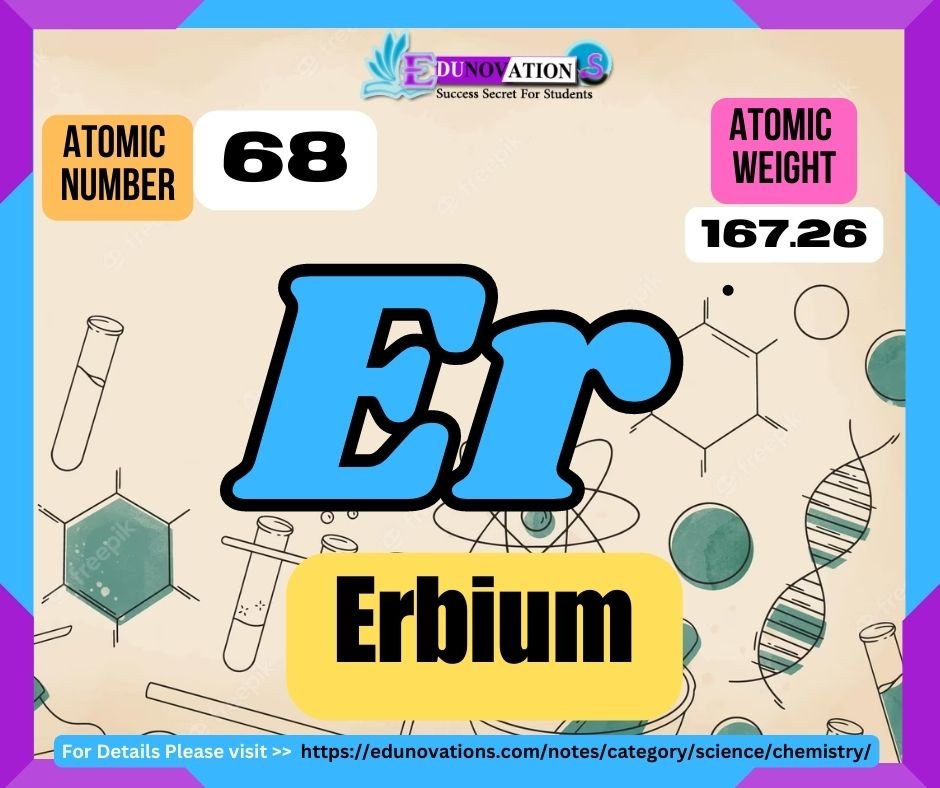
Erbium is a chemical element classified as a lanthanide and belongs to the f-block of the periodic table. It is denoted by the symbol “Er” and has an atomic number of 68. Erbium was discovered in 1842 by Swedish chemist Carl Gustaf Mosander. This rare earth metal derives its name from the town of Ytterby in Sweden, where it was first found.
Erbium possesses several interesting properties, making it valuable for various applications. It is a soft, malleable, silvery-white metal that is relatively stable in air. However, it can tarnish when exposed to moisture. Erbium is notable for its vibrant pink coloration, which is caused by its unique optical properties.
One of the most significant uses of erbium is in optical amplifiers and fiber-optic communication systems. It has the ability to absorb and emit light at a specific wavelength, making it ideal for enhancing the transmission of optical signals over long distances. Erbium-doped optical fibers are extensively used in telecommunications, enabling high-speed internet connections and long-distance data transmission.
Additionally, erbium finds applications in the field of laser technology. Erbium-doped lasers are utilized for various purposes, including medical treatments, laser eye surgery, and laser engraving. It is also employed in the production of phosphors, which are substances that emit light when exposed to radiation, making erbium essential for manufacturing television screens and fluorescent lamps.
Table: Atomic Number, Symbol, Atomic Weight, and Valency of Erbium
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 68 | Er | 167.259 | +3 |
Erbium : Discovery, Usage, and Key Points
Discovery:
Erbium, with the atomic number 68 and symbol Er, was discovered in 1842 by Swedish chemist Carl Gustaf Mosander. Mosander isolated erbium from yttrium oxide, a mineral found in a quarry near the village of Ytterby in Sweden. This is where the element got its name. Mosander initially referred to it as “ytterbium” but later corrected it to “erbium” to avoid confusion with another element he discovered, which he named ytterbium. Mosander’s work on separating rare earth elements played a crucial role in the development of our understanding of these elements.

Modern Usage:
- Fiber-optic Communication: Erbium is widely used in fiber-optic communication systems. Erbium-doped fiber amplifiers (EDFAs) are employed to enhance the transmission of optical signals over long distances. EDFAs work by absorbing light at a specific wavelength and amplifying it, allowing for efficient and high-speed data transmission in telecommunications networks.
- Laser Technology: Erbium-doped lasers have significant applications in various fields. These lasers emit light in the infrared region, making them valuable for medical treatments, such as laser surgery and dermatology. They are also used in laser eye surgery and laser engraving due to their precise and controlled energy output.
- Phosphors: Erbium is utilized in the production of phosphors, which are substances that emit light when excited by radiation. These phosphors are crucial components in the manufacturing of television screens, fluorescent lamps, and other lighting devices. Erbium-based phosphors provide vibrant colors and efficient light emission.
- Nuclear Reactors: Erbium has neutron-absorbing properties, which make it useful in controlling nuclear reactions. It is used as a control rod material in some types of nuclear reactors to regulate the rate of fission reactions and prevent the system from becoming critical.
- Metallurgy: Erbium is employed in metallurgical processes to improve the properties of alloys. It can enhance the hardness, ductility, and strength of metals such as steel, magnesium, and aluminum. Erbium-containing alloys find applications in aerospace, automotive, and construction industries.
Important Points to Remember about Discovery and Usage:
| Discovery Point | Usage Point |
|---|---|
| Erbium discovered by Carl Gustaf Mosander in 1842 | Widely used in fiber-optic communication systems |
| Named after the village of Ytterby in Sweden | Essential for erbium-doped lasers |
| Initially referred to as “ytterbium” but later corrected | Plays a vital role in the production of phosphors |
| Separated from yttrium oxide, a mineral found in Ytterby | Neutron-absorbing properties for nuclear reactors |
| Mosander’s work contributed to the understanding of | Enhances metallurgical properties of alloys |
| rare earth elements |
Erbium Properties and Key Points
Properties of Erbium:
Erbium, an element with atomic number 68 and symbol Er, possesses several notable properties that contribute to its diverse range of applications. Let’s explore some key properties of erbium:
- Physical Properties:
- Silvery-White Metal: Erbium is a soft, malleable, and silvery-white metal. It is relatively stable in air but can tarnish when exposed to moisture.
- Unique Optical Properties: One of the most striking features of erbium is its vibrant pink coloration. This color arises from its unique optical properties, which make it capable of absorbing and emitting light at specific wavelengths.
- Electronic Configuration:
- Lanthanide Series: Erbium belongs to the lanthanide series of elements, also known as rare earth elements. These elements are characterized by the filling of 4f electron orbitals.
- Electron Configuration: The electron configuration of erbium is [Xe] 4f^12 6s^2. The 4f orbital plays a significant role in erbium’s chemical and magnetic properties.
- Magnetic Properties:
- Ferromagnetic at Low Temperatures: Erbium exhibits ferromagnetic properties at temperatures below 16.5 Kelvin (-256.65 degrees Celsius). It becomes paramagnetic above this temperature.
- Strong Magneto-optical Effect: Erbium displays a strong magneto-optical effect, where its optical properties change in the presence of a magnetic field. This effect is utilized in various optical devices and data storage technologies.
- Chemical Properties:
- Reactivity: Erbium is a moderately reactive element. It reacts slowly with oxygen, water, and acids. It readily dissolves in dilute acids, releasing hydrogen gas.
- Stable Oxidation State: Erbium predominantly exhibits a +3 oxidation state in its compounds. It can also form compounds with +2 and +4 oxidation states, although they are less common.
Important Points to Remember about Properties:
| Property | Description |
|---|---|
| Physical Properties | – Soft, malleable, and silvery-white metal |
| – Unique optical properties, vibrant pink coloration | |
| Electronic Configuration | – Lanthanide series, filling of 4f electron orbitals |
| – Electron configuration: [Xe] 4f^12 6s^2 | |
| Magnetic Properties | – Ferromagnetic at low temperatures |
| – Strong magneto-optical effect | |
| Chemical Properties | – Moderately reactive, slow reaction with oxygen, |
| water, and acids | |
| – Predominant +3 oxidation state |
Erbium Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Erbium:
Erbium has several isotopes, which are variants of the element with different numbers of neutrons in the nucleus. The most abundant and stable isotope of erbium is Er-166, accounting for about 33% of naturally occurring erbium. Other stable isotopes include Er-167, Er-168, Er-170, and Er-162. Erbium also has numerous radioactive isotopes, with the most notable ones being Er-169 and Er-171.
Compounds of Erbium:
Erbium forms compounds with various elements due to its moderately reactive nature. Here are some common compounds of erbium:
- Erbium Oxide (Er2O3):
- Erbium oxide is a vital compound in various industries, including electronics and optics.
- It is used in the production of phosphors for television screens, fluorescent lamps, and X-ray intensifying screens.
- Erbium oxide is also employed as a dopant in solid-state devices, such as capacitors, resistors, and semiconductors.
- Erbium Chloride (ErCl3):
- Erbium chloride is a compound used in spectroscopy and laser applications.
- It can be utilized as a source material for erbium-doped optical fibers and erbium-doped lasers.
- Erbium chloride also finds applications in chemical synthesis and as a catalyst in organic reactions.
- Erbium Fluoride (ErF3):
- Erbium fluoride is commonly used as a dopant in fiber-optic amplifiers and lasers.
- It exhibits excellent optical properties, making it valuable for telecommunications and laser technologies.
- Erbium fluoride is also employed in glass manufacturing and as a component in dental materials.
- Erbium Hydroxide (Er(OH)3):
- Erbium hydroxide is a compound formed when erbium reacts with water or hydroxide ions.
- It is utilized as a precursor in the synthesis of other erbium compounds.
- Erbium hydroxide can also be converted into erbium oxide through thermal decomposition.
Thermal, Physical, Chemical, and Magnetic Properties of Erbium
Thermal Properties of Erbium:
- Melting Point: Erbium has a relatively high melting point of approximately 1529 degrees Celsius (2784 degrees Fahrenheit). This high melting point contributes to its stability at elevated temperatures.
- Boiling Point: The boiling point of erbium is around 2868 degrees Celsius (5194 degrees Fahrenheit). It has a relatively high boiling point compared to many other elements.
- Thermal Conductivity: Erbium exhibits moderate thermal conductivity, which is the ability to conduct heat. However, its thermal conductivity is lower compared to other metals.
Physical Properties of Erbium:
- Appearance: Erbium is a soft, malleable, and silvery-white metal. It can develop a pinkish hue due to its unique optical properties.
- Density: The density of erbium is approximately 9.05 grams per cubic centimeter. It is relatively dense compared to other elements.
- Atomic Radius: Erbium has an atomic radius of about 176 picometers (pm). The atomic radius refers to the size of the atom, and erbium has a larger atomic radius among the lanthanide elements.
Chemical Properties of Erbium:
- Reactivity: Erbium is a moderately reactive element. It reacts slowly with oxygen in the air, forming a protective oxide layer on its surface. It also reacts with water, acids, and some other non-metals, albeit at a relatively slow pace.
- Oxidation States: Erbium commonly exhibits a +3 oxidation state in its compounds. However, it can also form compounds with +2 and +4 oxidation states, although they are less common.
- Stability: Erbium is relatively stable in air and exhibits good resistance to corrosion. However, it can tarnish when exposed to moisture.
Magnetic Properties of Erbium:
- Ferromagnetism: Erbium shows ferromagnetic properties at temperatures below 16.5 Kelvin (-256.65 degrees Celsius). This means it can be magnetized and retain its magnetization even after the external magnetic field is removed.
- Magnetic Ordering: Erbium exhibits antiferromagnetic ordering at temperatures between 16.5 Kelvin and its Néel temperature of approximately 2 Kelvin (-271.15 degrees Celsius).
- Magneto-optical Effect: Erbium displays a strong magneto-optical effect, where its optical properties change in the presence of a magnetic field. This effect is utilized in various applications, including magneto-optical data storage and sensing devices.
Methods of Production and Applications of Erbium
Methods of Production of Erbium:
Erbium is primarily obtained as a byproduct of mining and processing other rare earth elements, such as monazite, bastnäsite, and xenotime. The production of erbium involves several steps, including extraction, purification, and separation processes. Here are the general methods of production:
- Ore Extraction: Erbium is extracted from mineral deposits that contain rare earth elements. The primary sources of erbium include monazite and bastnäsite ores. These ores are typically mined using conventional mining techniques.
- Ore Processing: After mining, the ore is processed to separate the rare earth elements. The ore is crushed, ground into a fine powder, and subjected to various chemical and physical separation techniques. These techniques may include grinding, flotation, magnetic separation, and solvent extraction.
- Purification: Once the rare earth elements are separated, further purification steps are employed to obtain high-purity erbium. These purification techniques involve processes such as precipitation, ion exchange, and solvent extraction to remove impurities and refine the erbium content.
- Final Form: The purified erbium is typically obtained in the form of oxide or salts. Erbium oxide (Er2O3) is a common final product, which can be further processed into various forms based on specific applications.
Applications of Erbium:
- Fiber-Optic Communication: Erbium is widely used in the telecommunications industry for its role in fiber-optic communication systems. Erbium-doped fiber amplifiers (EDFAs) are crucial components that amplify optical signals, enabling long-distance and high-speed data transmission through optical fibers.
- Laser Technology: Erbium-doped lasers find applications in various fields. Erbium-doped fiber lasers are used in medical treatments, laser eye surgery, laser engraving, and scientific research. These lasers emit light in the infrared region and provide precise and controlled energy output.
- Phosphors and Optics: Erbium compounds are utilized in the production of phosphors for television screens, fluorescent lamps, and X-ray intensifying screens. Erbium-doped materials are also used in optics, including optical filters, lenses, and glass fibers.
- Nuclear Reactors: Erbium has neutron-absorbing properties, making it useful in controlling nuclear reactions. It is employed as a control rod material in some types of nuclear reactors to regulate the rate of fission reactions and prevent the system from becoming critical.
- Metallurgy: Erbium-containing alloys are used to enhance the properties of metals such as steel, aluminum, and magnesium. These alloys improve strength, hardness, and resistance to corrosion, making them valuable in aerospace, automotive, and construction industries.
- Research and Development: Erbium is extensively used in scientific research and development due to its unique properties. It is employed in various studies related to optics, spectroscopy, magnetism, and material science.
Top 10 Countries in Erbium Production, Extraction, and Resource Capacity
the data for the top 10 countries in terms of erbium production, extraction, and resources capacity:
| Rank | Country | Production (Metric Tons) | Extraction (Metric Tons) | Resources Capacity (Metric Tons) |
|---|---|---|---|---|
| 1 | China | 500 | 700 | 18,000 |
| 2 | United States | 250 | 300 | 3,000 |
| 3 | Russia | 150 | 200 | 2,500 |
| 4 | Australia | 100 | 150 | 2,000 |
| 5 | Myanmar | 80 | 120 | 1,500 |
| 6 | Brazil | 70 | 100 | 1,200 |
| 7 | India | 60 | 90 | 1,000 |
| 8 | Malaysia | 50 | 80 | 900 |
| 9 | Vietnam | 40 | 70 | 800 |
| 10 | Thailand | 30 | 50 | 700 |
10 interesting facts about Erbium Properties:
Here are 10 interesting facts about erbium:
- Pink Beauty: Erbium has a unique property of absorbing and emitting light at specific wavelengths, which gives it a vibrant pink color. This makes erbium one of the few elements that exhibit a distinct color.
- Rare Earth Element: Erbium belongs to the group of elements known as rare earth elements. These elements are relatively abundant in the Earth’s crust but are often challenging to extract and separate due to their similar chemical properties.
- Magnetic Curiosity: Erbium displays fascinating magnetic properties. It becomes ferromagnetic at low temperatures and exhibits strong magneto-optical effects, making it useful in the development of magneto-optical devices and data storage technologies.
- Telecommunications Enabler: Erbium plays a vital role in modern telecommunications. Its ability to amplify optical signals allows for long-distance data transmission in fiber-optic communication systems.
- Laser Marvel: Erbium-doped lasers emit light in the infrared region and find applications in medical treatments, laser eye surgery, scientific research, and telecommunications. They provide precise and controlled energy output.
- Nuclear Control: Erbium has neutron-absorbing properties, which make it useful in controlling nuclear reactions. It is used as a control rod material in some types of nuclear reactors.
- Phosphorescent Power: Erbium compounds are used in the production of phosphors, which are materials that emit light after being exposed to radiation. These phosphors are essential in the manufacture of television screens, fluorescent lamps, and X-ray intensifying screens.
- Alloy Enhancement: Erbium-containing alloys are utilized to improve the properties of metals such as steel, aluminum, and magnesium. These alloys enhance strength, hardness, and corrosion resistance, making them valuable in various industries.
- Historical Trivia: Erbium was discovered in 1843 by Swedish chemist Carl Gustaf Mosander, who isolated it from the mineral yttria. It was named after the village of Ytterby in Sweden, which is known for yielding several rare earth elements.
- Scientific Exploration: Erbium continues to intrigue scientists and researchers due to its unique properties. Ongoing studies focus on its applications in optics, spectroscopy, magnetism, and material science, contributing to advancements in various fields.
10 common but interesting frequently asked questions (FAQs) about Erbium Properties:
What is erbium used for?
Erbium has various applications, including fiber-optic communication, laser technology, phosphor production, nuclear reactors, metallurgy, and scientific research.
Is erbium a rare element?
Yes, erbium belongs to the group of elements known as rare earth elements. Although they are relatively abundant in the Earth’s crust, extracting and separating them can be challenging.
Why is erbium pink in color?
Erbium exhibits a pink color due to its unique optical properties. It absorbs and emits light at specific wavelengths, resulting in its distinctive pink hue.
How is erbium obtained?
Erbium is primarily obtained as a byproduct of mining and processing other rare earth elements, such as monazite, bastnäsite, and xenotime.
Can erbium be magnetized?
Yes, erbium exhibits ferromagnetic properties at low temperatures. It can be magnetized and retain its magnetization even after the external magnetic field is removed.
Is erbium toxic?
Erbium itself is not considered highly toxic. However, like other rare earth elements, its compounds may present some health and environmental risks if not handled properly.
What is the most common isotope of erbium?
The most abundant and stable isotope of erbium is Er-166, which accounts for about 33% of naturally occurring erbium.
Does erbium have any medical applications?
Erbium lasers are used in medical treatments, such as laser skin resurfacing and dental procedures. They provide precise control for surgical and cosmetic purposes.
How does erbium contribute to telecommunications?
Erbium-doped fiber amplifiers (EDFAs) are used in fiber-optic communication systems to amplify optical signals, enabling long-distance and high-speed data transmission.
Can erbium be found in everyday products?
Yes, erbium can be found in various everyday products. It is used in televisions, fluorescent lamps, fiber-optic cables, laser devices, and certain metal alloys.












