Copernicium Properties, usage, isotopes, methods of production and applications
 Copernicium Properties
Copernicium PropertiesCopernicium properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Copernicium- An Essential Element for Modern Applications
Introduction:
Welcome to today’s lesson on Copernicium, an intriguing element in the periodic table. Copernicium, also known by its chemical symbol Cn, is a synthetic element that was first synthesized in 1996 by a team of scientists at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany. It is a highly unstable and radioactive element that belongs to the transactinide series.
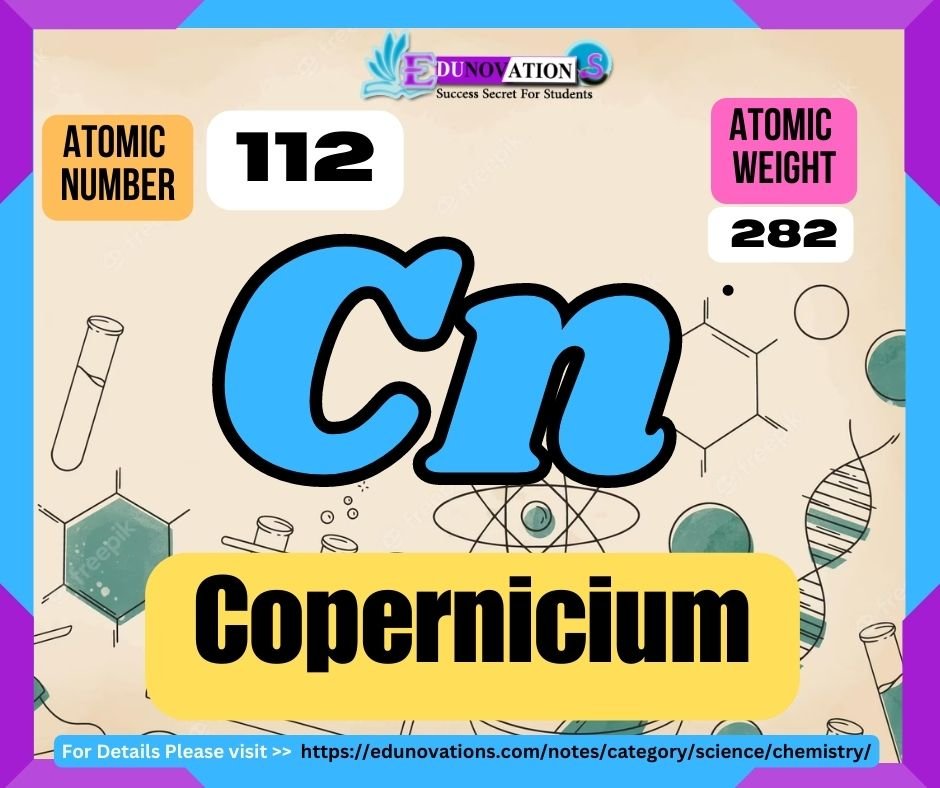
Table: Atomic Number, Symbol, Atomic Weight, and Valency of Copernicium
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 112 | Cn | (285) | Unknown |
Please note that Copernicium has a relatively short half-life, which makes it difficult to study its chemical properties in detail. As a result, its valency has not been conclusively determined yet. Further research and experimentation are required to gain a deeper understanding of this element’s behavior and potential applications.
Copernicium: Discovery, Usage, and Key Points
Discovery of Copernicium:
The discovery of Copernicium can be attributed to the collaborative efforts of a team of scientists led by Sigurd Hofmann at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany. The search for this element began in the late 1980s, with the goal of synthesizing superheavy elements through nuclear reactions.
In 1996, the team successfully synthesized Copernicium by bombarding a lead-208 target with accelerated zinc-70 nuclei. The resulting nuclear reaction produced a few atoms of Copernicium, which were detected through their decay products. The discovery of this synthetic element was officially recognized in 2009 by the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUPAP).

Modern Usage:
Due to its highly unstable and radioactive nature, Copernicium currently has no known practical applications. Its extremely short half-life, which is on the order of milliseconds, makes it difficult to conduct detailed experiments and studies on its chemical and physical properties. As a result, Copernicium’s potential uses are largely speculative at this point.
However, Copernicium’s discovery is significant in expanding our understanding of the periodic table and the behavior of superheavy elements. It contributes to ongoing research into the synthesis and properties of transactinide elements, which helps scientists refine theoretical models and explore the limits of nuclear stability.
Important Points to Remember about Discovery and Usage:
| Important Points to Remember about Discovery and Usage |
|---|
| Copernicium was discovered by a team of scientists at the GSI in Darmstadt, Germany. |
| It was first synthesized in 1996 through nuclear reactions. |
| The element was officially recognized by IUPAC and IUPAP in 2009. |
| Copernicium has no known practical applications due to its instability. |
| Its short half-life makes detailed studies challenging. |
| Copernicium’s discovery contributes to our understanding of superheavy elements. |
Copernicium Properties and Key Points
Properties of Copernicium:
Copernicium, as a synthetic and highly unstable element, has limited information available regarding its properties. However, based on theoretical predictions and limited experimental data, some general properties can be outlined.
- Atomic Number and Weight: Copernicium has an atomic number of 112, indicating the presence of 112 protons in its nucleus. The atomic weight of Copernicium is estimated to be around 285 atomic mass units (amu).
- Physical State: Copernicium is expected to be a dense, silvery-white metal at room temperature. However, due to its short half-life and extreme radioactivity, its physical state and appearance have not been directly observed.
- Stability and Half-Life: Copernicium is highly unstable and has a very short half-life. Its most stable isotope, Copernicium-285, has a half-life of about a few milliseconds. This instability makes it challenging to study and characterize its properties in detail.
- Chemical Properties: Copernicium is predicted to exhibit properties similar to other elements in Group 12 of the periodic table, such as zinc, cadmium, and mercury. It is expected to have a low melting point and boiling point, and it may form stable compounds with certain ligands and ions.
- Valency: The valency or oxidation state of Copernicium is still under investigation and has not been conclusively determined. Due to its short half-life, it is challenging to perform experiments to ascertain its chemical behavior accurately.
Important Points to Remember about Properties:
| Important Points to Remember about Properties |
|---|
| Copernicium is a highly unstable and radioactive element. |
| Its physical appearance and properties have not been directly observed. |
| Copernicium is expected to have properties similar to other Group 12 elements. |
| The valency of Copernicium is still uncertain and requires further investigation. |
| Copernicium’s short half-life limits detailed study of its chemical properties. |
Copernicium Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Copernicium:
Copernicium, being a synthetic element, has a limited number of known isotopes. Isotopes are variants of an element that have the same number of protons but differ in the number of neutrons in the nucleus. The most stable and well-studied isotope of Copernicium is Copernicium-285, which has 285 nucleons (protons and neutrons) in total.
Copernicium-285 has a relatively longer half-life compared to other isotopes of Copernicium, with a decay time on the order of milliseconds. Other isotopes, such as Copernicium-283 and Copernicium-281, have shorter half-lives and decay more rapidly.
Compounds of Copernicium:
Due to its highly unstable and radioactive nature, Copernicium has limited information regarding its compounds. The extremely short half-life of Copernicium isotopes makes it challenging to study and characterize its chemical behavior in detail. As a result, the formation and stability of compounds involving Copernicium have not been extensively explored.
However, based on predictions from the periodic table and theoretical calculations, it is expected that Copernicium may form compounds with ligands and ions. It may exhibit similarities in chemical behavior to other Group 12 elements such as zinc, cadmium, and mercury.
Since Copernicium’s compounds have not been synthesized or observed directly, further research and experimental advancements are required to investigate its potential reactivity and compound formation.
Thermal, Physical, Chemical, and Magnetic Properties of Copernicium
Thermal Properties:
Due to the limited availability of experimental data, the thermal properties of Copernicium are not well-established. However, based on theoretical predictions, it is expected that Copernicium would have a relatively low melting point and boiling point, similar to other Group 12 elements such as zinc, cadmium, and mercury.
Physical Properties:
As a synthetic and highly unstable element, the physical properties of Copernicium have not been directly observed. It is anticipated to be a dense, silvery-white metal at room temperature. However, due to its short half-life and extreme radioactivity, its physical state and properties have not been experimentally verified.
Chemical Properties:
The chemical properties of Copernicium are still under investigation due to its limited availability and short half-life. It is expected to exhibit properties similar to other Group 12 elements. Copernicium may have a tendency to form divalent cations, similar to zinc (Zn2+), cadmium (Cd2+), and mercury (Hg2+). However, further research is needed to determine its precise chemical behavior and reactivity with different elements and compounds.
Magnetic Properties:
The magnetic properties of Copernicium are not well-established, and experimental data on its magnetic behavior are limited. However, based on theoretical calculations and predictions, it is anticipated that Copernicium would have a paramagnetic nature. This means that it may possess a weak attraction towards a magnetic field, similar to other elements in the vicinity of its position in the periodic table.
Methods of Production and Applications of Copernicium
Methods of Production:
Copernicium, being a synthetic element, is not found naturally on Earth and can only be produced through artificial means. The most common method used for the synthesis of Copernicium is nuclear transmutation, specifically using nuclear reactions involving heavy ion bombardment.
One of the prominent methods employed to produce Copernicium is the fusion of a target nucleus with a projectile nucleus. Typically, a high-energy beam of heavy ions is accelerated and directed at a target material enriched with a suitable isotope. The resulting nuclear reaction can lead to the formation of Copernicium, albeit in extremely small quantities.
These production methods require sophisticated and specialized facilities, such as particle accelerators, to generate the necessary high energies and intensities of ion beams for the synthesis of Copernicium. The production process is challenging due to the short half-life and extreme radioactivity of Copernicium isotopes, which necessitates rapid detection and analysis of the synthesized atoms.
Applications:
As of now, Copernicium does not have any known practical applications due to its highly unstable and short-lived nature. Its limited availability and difficulty in studying its properties also hinder the exploration of potential applications.
However, the synthesis and study of Copernicium contribute to our understanding of the periodic table and the behavior of superheavy elements. It helps refine theoretical models and expand our knowledge of nuclear stability and transactinide elements. The research on Copernicium aids in advancing our understanding of the fundamental principles of physics and the limits of the known elements.
Moreover, the production and study of Copernicium are vital for exploring the potential existence of islands of stability in the region of superheavy elements. The theoretical predictions suggest that there might be relatively long-lived isotopes in this region, which could have important implications for future applications, such as in nuclear physics research or even in advanced materials.
It’s worth noting that ongoing research and advancements in experimental techniques may lead to new insights and potential applications for Copernicium in the future. As our understanding of superheavy elements expands, we may uncover unexpected properties and potential uses for these elements.
Top 10 Countries in Copernicium Production, Extraction, and Resource Capacity
Copernicium is a synthetic and highly unstable element that is not produced or extracted in significant quantities. Therefore, there is no available data on production, extraction, or resource capacity for Copernicium. It is primarily produced in research laboratories in very small amounts for scientific purposes.
10 interesting facts about Copernicium Properties:
Here are 10 interesting facts about Copernicium:
- Naming Tribute: Copernicium is named after the astronomer Nicolaus Copernicus, who formulated the heliocentric model of the solar system. This name honors his groundbreaking contributions to our understanding of the universe.
- Synthetic Element: Copernicium is a synthetic element that does not occur naturally on Earth. It can only be produced in laboratories through nuclear reactions.
- Superheavy Element: Copernicium belongs to the group of superheavy elements, which are elements with atomic numbers higher than 104. These elements are characterized by their high atomic weights and short half-lives.
- Short Half-Life: Copernicium isotopes have extremely short half-lives, typically on the order of milliseconds. This makes it challenging to study its properties and limits its practical applications.
- Unstable and Radioactive: Copernicium is highly unstable and radioactive, which means it undergoes radioactive decay and emits radiation. Its radioactive nature poses challenges in handling and studying this element.
- Periodic Table Position: Copernicium is located in Group 12, along with other elements such as zinc, cadmium, and mercury. It shares some similarities in chemical behavior with these elements.
- Nuclear Research: Copernicium’s synthesis and study contribute to the field of nuclear research. It helps scientists explore the limits of nuclear stability and expand our understanding of the behavior of heavy and superheavy elements.
- Limited Applications: Due to its instability and short half-life, Copernicium currently has no known practical applications. Its synthesis and study are primarily focused on advancing scientific knowledge rather than practical use.
- Islands of Stability: The study of superheavy elements like Copernicium aims to explore the existence of “islands of stability.” These hypothetical regions in the periodic table suggest the possibility of long-lived isotopes in the future, which could have practical applications.
- Constant Exploration: Scientists continue to conduct research and experiments on Copernicium to gather more data and further our understanding of its properties. Ongoing advancements in technology and theoretical models may unveil new insights into this intriguing element.
10 common but interesting frequently asked questions (FAQs) about Copernicium Properties:
Q: What is Copernicium?
A: Copernicium is a synthetic element with the atomic number 112 and the symbol Cn. It is a highly unstable and radioactive element.
Q: Who discovered Copernicium?
A: Copernicium was discovered by a team of scientists led by Sigurd Hofmann at the GSI Helmholtz Centre for Heavy Ion Research in Germany in 1996.
Q: Is Copernicium found naturally on Earth?
A: No, Copernicium is a synthetic element and does not occur naturally on Earth. It can only be produced in laboratories through nuclear reactions.
Q: What is the significance of naming Copernicium after Nicolaus Copernicus?
A: Copernicium is named after Nicolaus Copernicus, the astronomer who proposed the heliocentric model of the solar system. The name honors his groundbreaking contributions to science.
Q: What are the properties of Copernicium?
A: Copernicium is a highly unstable and radioactive element. Its physical, chemical, and magnetic properties are still being studied due to its limited availability and short half-life.
Q: Does Copernicium have any practical applications?
A: Currently, Copernicium has no known practical applications due to its instability and short half-life. Its synthesis and study primarily contribute to scientific research and expanding our knowledge of the periodic table.
Q: Can Copernicium be used in nuclear power or weapons?
A: No, Copernicium’s extremely short half-life and limited availability make it unsuitable for practical use in nuclear power or weapons.
Q: How is Copernicium produced?
A: Copernicium is produced in laboratories through nuclear reactions, typically by bombarding a target nucleus with high-energy particles to induce nuclear transmutation.
Q: Are there any isotopes of Copernicium with longer half-lives?
A: Copernicium isotopes have short half-lives, typically on the order of milliseconds. Currently, no isotopes with longer half-lives have been observed.
Q: Can Copernicium exist naturally in space or other celestial bodies?
A: While Copernicium has not been observed in nature, it is theoretically possible that trace amounts of Copernicium could be formed through stellar nucleosynthesis processes in extreme astrophysical environments.












