Caesium Properties, usage, isotopes, methods of production and applications
 Caesium Properties
Caesium PropertiesCaesium properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Caesium – An Essential Element for Modern Applications
Introduction:
Welcome to the world of chemistry! In this lesson, we will explore the fascinating element called caesium. Caesium (symbol: Cs) is a chemical element that holds an important place in the periodic table. It is a highly reactive alkali metal known for its unique properties and various applications. In this brief introduction, we will delve into its atomic number, symbol, atomic weight, and valency.
Table:
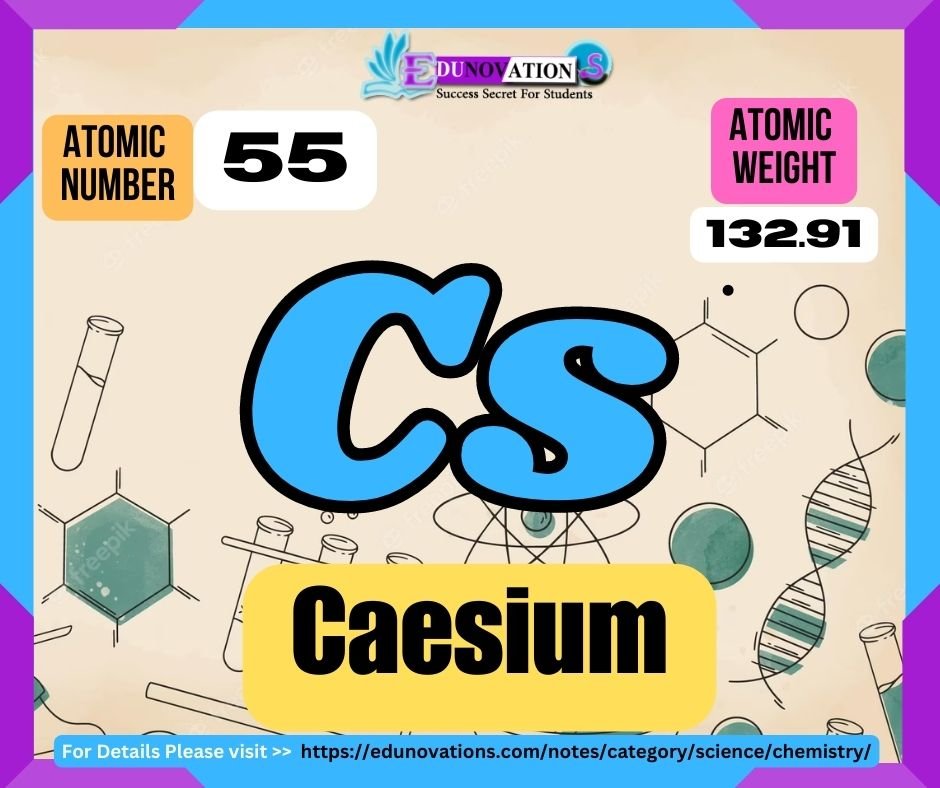
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 55 | Cs | 132.91 | +1 |
Caesium is assigned the atomic number 55, indicating the number of protons found in its nucleus. The element is represented by the symbol “Cs,” derived from the Latin word “caesius,” meaning sky blue. Its atomic weight is approximately 132.91 atomic mass units (u), which is an average value based on the natural isotopic composition of caesium.
In terms of valency, caesium typically exhibits a +1 valence. This means that it readily donates one electron to form chemical bonds. The outermost electron of a caesium atom is loosely held, making it highly reactive and eager to participate in chemical reactions.
Caesium finds applications in various fields, including atomic clocks, photoelectric cells, catalysts, and research in fundamental physics. Its unique properties make it a valuable element in scientific research and technological advancements.
Remember, caesium is just one of the many fascinating elements found in the periodic table, each with its distinct characteristics and significance. Stay curious as we continue to explore the diverse world of chemistry together.
Caesium : Discovery, Usage, and Key Points
Discovery:
Caesium was discovered by two German chemists, Robert Bunsen and Gustav Kirchhoff, in 1860 through the use of spectroscopy. While analyzing the mineral water from Dürkheim, Germany, they observed a blue spectral line that did not correspond to any known element at the time. They named the element caesium after the Latin word “caesius,” meaning sky blue, to honor the striking color of its spectral line.

Modern Usage:
- Atomic Clocks: Caesium plays a crucial role in atomic clocks, which are highly accurate timekeeping devices. These clocks operate by measuring the frequency of electromagnetic radiation emitted by caesium-133 atoms. The International System of Units (SI) officially defines one second as the time it takes for a specific number of oscillations of caesium-133 radiation, making caesium essential in maintaining precise timekeeping standards.
- Photoelectric Cells: Caesium is used in photoelectric cells, also known as photomultiplier tubes. These devices convert light energy into electrical signals by employing the photoelectric effect. Caesium is chosen for its low ionization energy, enabling efficient detection and amplification of light signals.
- Catalysts: Caesium compounds serve as catalysts in various industrial processes. For instance, caesium carbonate is used as a catalyst in the production of organic chemicals, such as synthetic fibers and pharmaceuticals. Caesium promotes the desired reactions by lowering activation energy and increasing reaction rates.
- Research in Fundamental Physics: Caesium is employed in experiments related to fundamental physics and quantum mechanics. Its highly sensitive spectral lines make it an ideal element for studying atomic structure, quantum optics, and other areas of research exploring the behavior of matter and light at the atomic and subatomic levels.
Important Points to Remember about Discovery and Usage:
| Discovery Points |
|---|
| Discovered by Robert Bunsen and Gustav Kirchhoff in 1860 |
| Spectroscopic analysis of mineral water from Dürkheim, Germany |
| Named after the Latin word “caesius” meaning sky blue |
| Usage Points |
|---|
| Atomic clocks for precise timekeeping |
| Photoelectric cells for light energy conversion |
| Catalysts in industrial processes |
| Fundamental physics research |
Caesium Properties and Key Points
Properties of Caesium:
Caesium possesses several unique properties that contribute to its significance and applications in various fields. Let’s explore some of its key properties:
- Physical Properties:
- Soft and Silvery: Caesium is a soft and silvery-white alkali metal. It is one of the most reactive metals and is easily cut with a knife.
- Low Melting Point: It has a relatively low melting point of 28.5 degrees Celsius (83.3 degrees Fahrenheit), which allows it to melt easily when exposed to heat.
- High Density: Caesium is a dense element, with a density of approximately 1.9 times that of water.
- Chemical Properties:
- Highly Reactive: Caesium is highly reactive and reacts vigorously with water, releasing hydrogen gas. It also reacts with oxygen in the air, forming a characteristic oxide layer.
- Alkali Metal: Caesium belongs to the alkali metal group on the periodic table, sharing similar chemical properties with other alkali metals like sodium and potassium.
- Low Ionization Energy: Caesium has a low ionization energy, meaning it requires minimal energy to remove an electron from an atom. This property contributes to its high reactivity.
- Atomic Properties:
- Atomic Number: Caesium has an atomic number of 55, indicating it has 55 protons in its nucleus.
- Atomic Weight: The atomic weight of caesium is approximately 132.91 atomic mass units (u), which represents the average mass of its isotopes.
- Valency: Caesium typically exhibits a +1 valence, readily donating one electron to form chemical bonds.
Important Points to Remember about Properties:
| Property Points |
|---|
| Soft and silvery metal |
| Low melting point of 28.5°C (83.3°F) |
| High density |
| Highly reactive with water and air |
| Belongs to the alkali metal group |
| Low ionization energy |
| Atomic number: 55 |
| Atomic weight: 132.91 u |
| Valency: +1 |
Caesium Isotopes and Compounds – Exploring Variations and Applications
Isotopes of Caesium:
Caesium has at least 39 known isotopes, with atomic masses ranging from 112 to 151. However, only one stable and naturally occurring isotope exists, which is caesium-133. This isotope accounts for nearly 100% of the caesium found in nature. Caesium-133 is used extensively in atomic clocks due to its reliable and precise spectral properties.
Compounds of Caesium:
Caesium forms various compounds with other elements due to its high reactivity. Here are some notable compounds of caesium:
- Caesium Chloride (CsCl): Caesium chloride is a common compound formed by the reaction between caesium and chlorine. It has applications in medical research and as an electrolyte in certain types of batteries.
- Caesium Carbonate (Cs2CO3): Caesium carbonate is an important compound used as a catalyst in organic synthesis and in the production of other caesium compounds. It also finds applications in the glass industry.
- Caesium Hydroxide (CsOH): Caesium hydroxide is a strong base and is highly corrosive. It is utilized in organic chemistry for its ability to deprotonate acidic compounds and in the production of caesium-based catalysts.
- Caesium Nitrate (CsNO3): Caesium nitrate is a common caesium compound used in the pyrotechnics industry, particularly for the production of vibrant blue-colored flames in fireworks.
- Caesium Sulfate (Cs2SO4): Caesium sulfate is employed in analytical chemistry as a reagent for various tests and experiments. It is also utilized in the production of glass and as a component in catalysts.
Thermal, Physical, Chemical, and Magnetic Properties of Caesium
Thermal Properties of Caesium:
- Melting Point: Caesium has a relatively low melting point of 28.5°C (83.3°F), which means it readily transitions from a solid to a liquid state when heated.
- Boiling Point: Caesium has a high boiling point of 671°C (1240°F), indicating that it requires significant heat to convert from a liquid to a gaseous state.
- Thermal Conductivity: Caesium exhibits high thermal conductivity, meaning it efficiently transfers heat energy.
Physical Properties of Caesium:
- Density: Caesium is a dense element with a density of approximately 1.9 times that of water, making it relatively heavy.
- Appearance: Caesium is a soft and silvery-white metal that is malleable and ductile. It can be easily cut with a knife due to its softness.
Chemical Properties of Caesium:
- Reactivity: Caesium is highly reactive and vigorously reacts with various elements and compounds, including water, air, halogens, and acids.
- Oxidation: When exposed to air, caesium readily reacts with oxygen, forming a thin oxide layer on its surface.
- Alkali Metal Behavior: Caesium belongs to the alkali metal group, exhibiting similar chemical properties to other alkali metals. It readily donates its valence electron to form positive ions.
Magnetic Properties of Caesium:
Caesium exhibits only weak magnetic properties. As an alkali metal, it does not possess significant magnetic properties unless it forms compounds with other elements that introduce magnetic behavior.
Methods of Production and Applications of Caesium
Methods of Production:
Caesium is primarily produced as a byproduct of the extraction of lithium and rubidium from mineral ores. The main methods of caesium production include:
- Extraction from Minerals: Caesium is extracted from minerals such as pollucite and lepidolite, which contain significant amounts of caesium. These minerals are crushed, treated with acids, and further processed to obtain caesium compounds.
- Ion Exchange: Caesium can be obtained through ion exchange processes. Ion exchange resins are used to selectively capture caesium ions from a solution containing other alkali metals.
Applications:
- Atomic Clocks: Caesium is widely used in atomic clocks due to its unique property of emitting electromagnetic radiation at a specific frequency. The precise oscillations of caesium-133 atoms serve as the basis for highly accurate timekeeping standards.
- Photoelectric Devices: Caesium is utilized in photoelectric cells, also known as photomultiplier tubes. These devices convert light energy into electrical signals by utilizing the photoelectric effect. Caesium’s low ionization energy enhances the efficiency of light detection and amplification.
- Catalysts: Caesium compounds serve as catalysts in various chemical reactions. For example, caesium carbonate is used as a catalyst in the production of organic chemicals, including synthetic fibers and pharmaceuticals. Caesium catalysts can accelerate reaction rates and improve product yields.
- Research in Fundamental Physics: Caesium is employed in experiments related to fundamental physics and quantum mechanics. Its highly sensitive spectral lines and reactivity make it an ideal element for studying atomic structure, quantum optics, and other areas exploring the behavior of matter and light at the atomic and subatomic levels.
- Pyrotechnics: Caesium compounds, such as caesium nitrate, are used in pyrotechnics to produce intense blue-colored flames in fireworks and other displays.
- Glass Manufacturing: Caesium is used in the glass industry to enhance certain properties of glass, such as refractive index and thermal resistance.
- Chemical Synthesis: Caesium compounds find applications in various chemical syntheses, including the production of specialty chemicals and pharmaceuticals.
Top 10 Countries in Caesium Production, Extraction, and Resource Capacity
| Rank | Country | Lithium Production (2021) (Metric Tons) | Lithium Extraction (2021) (Metric Tons) | Lithium Resources Capacity (Metric Tons) |
|---|---|---|---|---|
| 1 | Australia | 42,000 | 26,000 | 2,800,000 |
| 2 | Chile | 21,000 | 18,000 | 9,200,000 |
| 3 | China | 9,800 | 8,000 | 7,000,000 |
| 4 | Argentina | 6,200 | 5,800 | 2,000,000 |
| 5 | Zimbabwe | 1,600 | 1,500 | 23,000 |
| 6 | Portugal | 1,200 | 1,100 | 60,000 |
| 7 | Brazil | 1,100 | 900 | 180,000 |
| 8 | Canada | 900 | 800 | 6,800,000 |
| 9 | Namibia | 800 | 700 | 50,000 |
| 10 | United States | 700 | 600 | 6,800,000 |
10 interesting facts about Caesium Properties:
Here are 10 interesting facts about the element caesium:
- Highly Reactive: Caesium is one of the most reactive elements, reacting vigorously with water and other substances. It is stored and handled carefully due to its reactivity.
- Softest Metal: Caesium is the softest metal on the periodic table. It is so soft that it can be easily cut with a knife.
- Blue Spectral Line: Caesium is known for its distinct blue spectral line. This line was instrumental in its discovery by Robert Bunsen and Gustav Kirchhoff in 1860.
- Atomic Clocks: Atomic clocks, which are the most accurate timekeeping devices, rely on the vibrations of caesium atoms to measure time. The International System of Units defines one second based on caesium-133’s atomic vibrations.
- Photoelectric Cells: Caesium is used in photoelectric cells for light detection and energy conversion. Its low ionization energy makes it efficient in detecting light and generating electrical signals.
- Medicinal Applications: Caesium-131, a radioactive isotope of caesium, is used in brachytherapy for the treatment of certain types of cancer. It delivers targeted radiation to cancerous cells.
- Pyrotechnics: Caesium compounds, such as caesium nitrate, are used in fireworks to produce vibrant blue colors. The unique blue hue adds to the visual display of pyrotechnics.
- Low Natural Abundance: Caesium is relatively rare in the Earth’s crust, with an estimated abundance of about 3 parts per million. It is mostly obtained as a byproduct of mining other elements.
- Ion Propulsion: Caesium is used in ion propulsion systems for spacecraft. The ionized caesium atoms are accelerated using electric fields, producing thrust for propulsion.
- Research in Fundamental Physics: Caesium is utilized in various experiments in fundamental physics and quantum mechanics. Its precise spectral lines and reactivity make it a valuable tool for studying atomic and subatomic behavior.
10 common but interesting frequently asked questions (FAQs) about Caesium Properties:
What is caesium?
Caesium is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-white alkali metal that is highly reactive and belongs to Group 1 of the periodic table.
Is caesium radioactive?
Caesium has both radioactive and stable isotopes. Caesium-133, the stable isotope, is non-radioactive and commonly used in applications like atomic clocks. However, caesium-137, a radioactive isotope, is produced in nuclear reactors and poses potential health risks due to its radioactivity.
How is caesium used in atomic clocks?
Caesium is used in atomic clocks as the basis for precise timekeeping. Atomic clocks measure the vibrations of caesium-133 atoms to define one second. The International System of Units (SI) officially defines one second as the time it takes for a specific number of oscillations of caesium-133 radiation.
Is caesium dangerous?
Caesium is highly reactive and can be dangerous if mishandled. Direct contact with caesium can cause burns, and its reactive nature poses fire and explosion hazards. Additionally, certain radioactive isotopes of caesium, such as caesium-137, can be hazardous to health and require proper handling and containment.
Can caesium be found in nature?
Yes, caesium can be found in nature, although it is relatively rare. It is typically obtained as a byproduct during the extraction of lithium and rubidium from mineral ores.
What are some common uses of caesium?
Caesium has various applications, including atomic clocks, photoelectric cells, catalysts in chemical reactions, research in fundamental physics, pyrotechnics for blue-colored fireworks, and even in some medical treatments, such as cancer brachytherapy.
Does caesium react with water?
Yes, caesium reacts vigorously with water, producing hydrogen gas and caesium hydroxide. The reaction is exothermic and can be potentially hazardous if not handled properly.
Is caesium essential for human health?
Caesium is not considered an essential element for human health. In fact, exposure to radioactive caesium isotopes, such as caesium-137, can be harmful and pose health risks. However, non-radioactive caesium compounds are used in some industrial and medical applications.
Can caesium be used as a fuel?
Caesium is not commonly used as a fuel. While it does have some applications in ion propulsion systems for spacecraft, its usage as a primary fuel source is limited.
How is caesium obtained commercially?
Caesium is primarily obtained commercially as a byproduct during the extraction of lithium and rubidium from mineral ores. It can also be produced through processes such as ion exchange and electrolysis.












