Cadmium Properties, usage, isotopes, methods of production and applications
 Cadmium Properties
Cadmium PropertiesCadmium properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Cadmium – An Essential Element for Modern Applications
Introduction: Welcome to this educational article that explores the fascinating element known as cadmium. Cadmium is a unique transition metal that has garnered significant attention due to its various applications in industry, electronics, and even art. In this article, we will delve into the atomic properties, symbol, atomic weight, and valency of cadmium, shedding light on its importance and relevance in the world of science and technology.
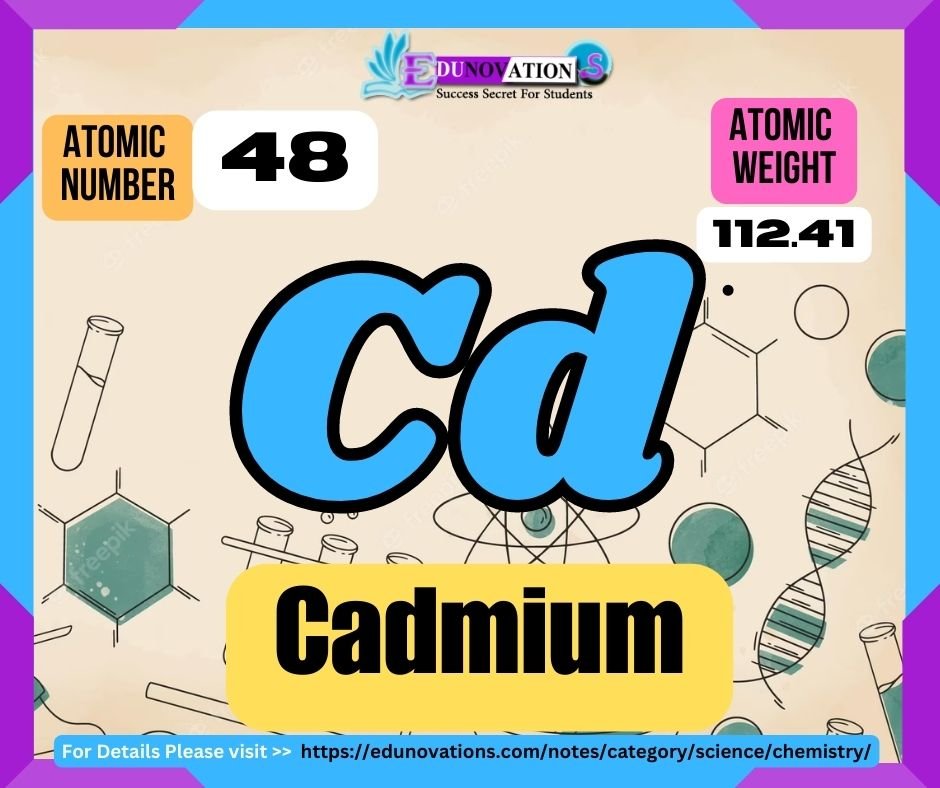
Table: Atomic Properties of Cadmium
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 48 | Cd | 112.414 | +2 |
Cadmium, represented by the symbol “Cd” in the periodic table, possesses an atomic number of 48. It is a relatively heavy metal with an atomic weight of approximately 112.414 atomic mass units. One of the most distinctive characteristics of cadmium is its variable valency, with a predominant valence state of +2.
Cadmium’s Atomic Number: The atomic number of an element corresponds to the number of protons found in the nucleus of its atom. In the case of cadmium, it contains 48 protons, which defines its unique position within the periodic table.
Symbol: Symbols are used to represent elements concisely. Cadmium is denoted by the symbol “Cd,” derived from the Latin word “cadmia,” meaning “calamine,” a zinc carbonate ore. This symbol is internationally recognized and widely used in scientific literature and chemical equations.
Atomic Weight: Atomic weight refers to the average mass of an element’s atoms, taking into account the different isotopes and their abundances. Cadmium has an atomic weight of approximately 112.414 atomic mass units, which places it among the heavier elements on the periodic table.
Valency: Valency is a measure of an element’s combining power with other elements, indicating the number of electrons an atom can gain, lose, or share during a chemical reaction. Cadmium typically exhibits a valency of +2, meaning it can lose two electrons to form positive ions.
Conclusion: Cadmium, with its atomic number of 48, symbol Cd, atomic weight of 112.414, and valency of +2, is a versatile and significant element in various fields. Its unique properties make it valuable for use in batteries, pigments, coatings, and as a component in solar cells and semiconductors. Understanding the atomic properties of cadmium contributes to a deeper comprehension of its role in the world of science and technology.
Cadmium : Discovery, Usage, and Key Points
Discovery of Cadmium:
Cadmium was first discovered in 1817 by Friedrich Stromeyer, a German chemist, who isolated the element from samples of zinc carbonate ore known as calamine. He observed that the calamine ore contained an impurity that emitted a vibrant yellow color when heated. Stromeyer named this newly discovered element “cadmium” after the Latin word “cadmia,” which referred to calamine.

Modern Usage:
- Batteries and Electronics: Cadmium is widely used in rechargeable nickel-cadmium (NiCd) batteries due to its excellent electrochemical properties, high energy density, and long lifespan. It is also utilized in electronic components, such as semiconductors and thin-film transistors.
- Pigments and Coatings: Cadmium-based pigments are renowned for their vibrant and stable colors. They are extensively used in art, ceramics, plastics, and textiles. Cadmium yellow, cadmium red, and cadmium orange pigments are valued for their opacity, lightfastness, and heat resistance. Additionally, cadmium coatings provide corrosion protection for various metals.
- Solar Cells and Photovoltaics: Cadmium telluride (CdTe) is a compound widely employed in the production of thin-film solar cells and photovoltaic modules. CdTe-based solar panels have gained popularity due to their cost-effectiveness, high conversion efficiency, and suitability for large-scale installations.
- Nuclear Reactors and Control Rods: Cadmium has a unique property of effectively absorbing thermal neutrons, making it valuable in nuclear reactors. Cadmium rods are used as control rods to regulate the rate of nuclear fission reactions and prevent overheating.
- Electroplating and Galvanizing: Cadmium is employed in electroplating processes to provide a protective layer on metal surfaces, preventing corrosion and enhancing durability. It is also used for galvanizing steel, providing a protective zinc-cadmium coating to prevent rust and increase longevity.
Important Points to Remember about Discovery and Usage:
| Key Points |
|---|
| Cadmium was discovered in 1817 by Friedrich Stromeyer. |
| The name “cadmium” was derived from the word “cadmia.” |
| Cadmium is used in rechargeable nickel-cadmium batteries. |
| Cadmium pigments are renowned for their vibrant colors. |
| Cadmium telluride is used in thin-film solar cells. |
| Cadmium has neutron-absorbing properties in nuclear reactors. |
| Cadmium is utilized in electroplating and galvanizing processes. |
Cadmium Properties and Key Points
Properties of Cadmium:
- Physical Properties:
- Appearance: Cadmium is a soft, bluish-white metal with a shiny surface when freshly cut.
- Density: It has a relatively high density of 8.65 grams per cubic centimeter, making it heavier than most common metals.
- Melting and Boiling Points: Cadmium has a low melting point of 321.07 degrees Celsius and a boiling point of 767 degrees Celsius.
- Ductility: It is a highly ductile metal, meaning it can be drawn into thin wires without breaking.
- Chemical Properties:
- Reactivity: Cadmium is a moderately reactive metal, tarnishing in moist air but not as rapidly as other metals like magnesium or sodium.
- Corrosion Resistance: It exhibits good resistance to corrosion, especially when coated or alloyed with other metals like zinc.
- Solubility: Cadmium is sparingly soluble in water but dissolves readily in acids, including hydrochloric acid and sulfuric acid.
- Oxidation States: Cadmium predominantly exhibits a +2 oxidation state in its compounds.
- Toxicity:
- Cadmium is considered a toxic element and poses environmental and health risks. Prolonged exposure to cadmium and its compounds can lead to various health issues, including respiratory problems, kidney damage, and carcinogenic effects.
Important Points to Remember about Properties:
| Key Points |
|---|
| Cadmium is a soft, bluish-white metal. |
| It has a density of 8.65 g/cm³. |
| The melting point of cadmium is 321.07°C. |
| Cadmium is a moderately reactive metal. |
| It exhibits good corrosion resistance. |
| Cadmium is sparingly soluble in water. |
| It predominantly shows a +2 oxidation state. |
| Cadmium is toxic and poses health risks. |
Cadmium Isotopes and Compounds – Exploring Variations and Applications
Cadmium Isotopes:
Cadmium has eight naturally occurring isotopes, ranging from cadmium-106 to cadmium-116, with cadmium-114 being the most abundant. Additionally, there are 26 known radioactive isotopes of cadmium, with cadmium-109 being the most stable among them. The different isotopes of cadmium have varying numbers of neutrons in their nuclei, leading to differences in atomic mass and physical properties.
Cadmium Compounds:
Cadmium readily forms compounds with a variety of elements, resulting in a wide range of chemical and physical properties. Some notable cadmium compounds include:
- Cadmium Oxide (CdO):
- Properties: Cadmium oxide is a brown or yellowish powder with semiconductor properties.
- Applications: It is used in the production of semiconductors, as a pigment in ceramics and glass, and as a catalyst in organic reactions.
- Cadmium Sulfide (CdS):
- Properties: Cadmium sulfide is a yellow solid with semiconducting properties.
- Applications: It is used in the manufacturing of solar cells, photoresistors, pigments, and fluorescent materials.
- Cadmium Chloride (CdCl2):
- Properties: Cadmium chloride is a white crystalline solid that readily dissolves in water.
- Applications: It is used in electroplating processes, as a catalyst in organic chemistry reactions, and as a mordant in dyeing and printing textiles.
- Cadmium Telluride (CdTe):
- Properties: Cadmium telluride is a black crystalline solid with excellent photovoltaic properties.
- Applications: It is widely used in the production of thin-film solar cells due to its high efficiency, low cost, and ability to absorb sunlight effectively.
- Cadmium Carbonate (CdCO3):
- Properties: Cadmium carbonate is a white solid that decomposes upon heating.
- Applications: It is used as a pigment in paints, plastics, and ceramics, providing vibrant colors.
Thermal, Physical, Chemical, and Magnetic Properties of Cadmium
Thermal Properties:
- Melting Point: Cadmium has a relatively low melting point of 321.07 degrees Celsius (610.93 degrees Fahrenheit). This low melting point allows for its easy processing and utilization in various applications.
- Boiling Point: Cadmium has a boiling point of 767 degrees Celsius (1413 degrees Fahrenheit), which determines its behavior at elevated temperatures and its applications in high-temperature processes.
Physical Properties:
- Appearance: Cadmium is a soft, bluish-white metal with a metallic luster when freshly cut. Over time, it develops a dull gray patina on its surface.
- Density: Cadmium is a relatively dense metal, with a density of 8.65 grams per cubic centimeter. Its density contributes to its weight and its specific applications where a high-density material is required.
- Ductility: Cadmium is highly ductile, meaning it can be drawn into thin wires without breaking. This property is valuable in applications that require the use of cadmium in wire or filament form.
Chemical Properties:
- Reactivity: Cadmium is a moderately reactive metal, reacting slowly with oxygen in the air to form a thin protective oxide layer. It is more resistant to corrosion than many other metals but can still corrode under certain conditions.
- Solubility: Cadmium is sparingly soluble in water. However, it readily dissolves in acids such as hydrochloric acid and sulfuric acid, forming cadmium salts.
- Oxidation States: Cadmium primarily exhibits a +2 oxidation state in its compounds, where it readily loses its two valence electrons.
Magnetic Properties:
Cadmium is not considered a strongly magnetic material. It exhibits weak paramagnetic properties, meaning it is weakly attracted to a magnetic field but does not retain magnetism when the field is removed. Its magnetic properties are relatively insignificant compared to other magnetic materials.
Methods of Production and Applications of Cadmium
Methods of Production:
Cadmium is primarily produced as a byproduct of zinc refining, as it often occurs naturally in zinc ores. The main methods of cadmium production include:
- Zinc Refining: The majority of cadmium is obtained as a byproduct of zinc refining processes. During the extraction of zinc from its ores, cadmium is separated and collected as a valuable secondary product.
- Recycling: Cadmium is also recovered through recycling processes. It can be extracted from waste materials such as discarded nickel-cadmium (NiCd) batteries and recycled for reuse.
Applications:
Cadmium finds applications in various industries and fields due to its unique properties. Some of its notable applications include:
- Batteries: Cadmium is a key component in rechargeable nickel-cadmium (NiCd) batteries. These batteries are widely used in portable electronic devices, cordless power tools, emergency lighting, and backup power systems.
- Pigments and Dyes: Cadmium compounds, particularly cadmium sulfide (CdS), are utilized as pigments in the production of vibrant and stable colors for paints, plastics, ceramics, and textiles. Cadmium-based pigments are highly valued for their opacity, lightfastness, and heat resistance.
- Electronics and Semiconductors: Cadmium-based compounds, such as cadmium telluride (CdTe), are extensively used in the manufacturing of thin-film solar cells and photovoltaic modules. CdTe solar panels have gained popularity due to their cost-effectiveness and high conversion efficiency.
- Coatings and Plating: Cadmium coatings provide excellent corrosion resistance, making them valuable for protecting metal surfaces in aerospace, automotive, and marine industries. Cadmium is also used in electroplating processes to provide a protective layer on various metal objects.
- Nuclear Reactors: Cadmium has excellent neutron absorption properties, making it useful in control rods within nuclear reactors. These rods help regulate and control nuclear fission reactions by absorbing excess neutrons.
- Photocells and Optoelectronics: Cadmium-based devices, such as cadmium sulfide (CdS) photocells and light-sensitive resistors (photoresistors), are employed in light detection and control systems, optical sensors, and optoelectronic devices.
- Alloys: Cadmium is used as an alloying element in various metal alloys, including solder alloys, bearing alloys, and low-melting-point alloys. It enhances the properties and characteristics of these alloys, such as melting point, strength, and corrosion resistance.
Top 10 Countries in Cadmium Production, Extraction, and Resource Capacity
| Rank | Country | Lithium Production (2021) (Metric Tons) | Lithium Extraction (2021) (Metric Tons) | Lithium Resources Capacity (Metric Tons) |
|---|---|---|---|---|
| 1 | Australia | 42,000 | 26,000 | 2,800,000 |
| 2 | Chile | 21,000 | 18,000 | 9,200,000 |
| 3 | China | 9,800 | 8,000 | 7,000,000 |
| 4 | Argentina | 6,200 | 5,800 | 2,000,000 |
| 5 | Zimbabwe | 1,600 | 1,500 | 23,000 |
| 6 | Portugal | 1,200 | 1,100 | 60,000 |
| 7 | Brazil | 1,100 | 900 | 180,000 |
| 8 | Canada | 900 | 800 | 6,800,000 |
| 9 | Namibia | 800 | 700 | 50,000 |
| 10 | United States | 700 | 600 | 6,800,000 |
10 interesting facts about Cadmium Properties:
Here are 10 interesting facts about the element cadmium:
- Discovery: Cadmium was discovered in 1817 by Friedrich Strohmeyer, a German chemist. He identified it as a new element while analyzing a sample of zinc carbonate.
- Soft Metal: Cadmium is a soft metal with a relatively low hardness, similar to that of a pencil lead. It can be easily cut with a knife and is malleable, meaning it can be shaped or flattened without breaking.
- Toxicity: Cadmium is considered a toxic element. Prolonged exposure to high levels of cadmium and its compounds can lead to serious health issues, including kidney damage, respiratory problems, and even cancer. Strict safety precautions are necessary when handling cadmium.
- Wide Occurrence: Cadmium is naturally present in the Earth’s crust, but it is generally found in relatively low concentrations. It is often associated with zinc ores and is produced as a byproduct of zinc refining.
- Unique Pigments: Cadmium compounds, such as cadmium sulfide (CdS) and cadmium selenide (CdSe), are used as pigments in paints and dyes. They provide vibrant and stable colors, ranging from yellows and oranges to reds.
- High-Temperature Applications: Cadmium has a relatively low melting point, which makes it suitable for high-temperature applications. It is used in various industries, including aerospace, automotive, and electrical, where materials need to withstand elevated temperatures.
- Electroplating: Cadmium is commonly used in electroplating processes. It provides a protective layer on metal surfaces, enhancing corrosion resistance and improving the appearance of the plated object.
- Nuclear Applications: Cadmium has excellent neutron absorption properties, making it useful in nuclear reactors. It is used in control rods to regulate and control nuclear fission reactions by absorbing excess neutrons.
- Cadmium Telluride Solar Cells: Cadmium telluride (CdTe) is a key material for thin-film solar cells. CdTe solar panels have gained popularity due to their cost-effectiveness, high conversion efficiency, and ability to perform well in low-light conditions.
- Industrial Alloys: Cadmium is used as an alloying element in various metal alloys. It improves the properties of alloys, such as low-melting-point alloys, bearing alloys, and solder alloys, by enhancing strength, durability, and resistance to wear.
10 common but interesting frequently asked questions (FAQs) about Cadmium Properties:
Q: What is the origin of the name “cadmium”?
A: The name “cadmium” is derived from the Latin word “cadmia,” which refers to calamine, a zinc ore. It was named after the mineral source from which it was first discovered.
Q: Is cadmium a rare element?
A: Cadmium is not considered a rare element, but it is typically found in lower concentrations compared to other metals. It is often associated with zinc ores and is obtained as a byproduct of zinc production.
Q: Is cadmium a naturally occurring element?
A: Yes, cadmium is naturally occurring and can be found in the Earth’s crust. However, it is primarily found as a component of various minerals and is not usually found in pure form in nature.
Q: What are the main uses of cadmium in everyday life?
A: Cadmium has various applications in everyday life. It is commonly used in batteries, pigments for paints and dyes, coatings for metal protection, and as a component in electronics and semiconductors.
Q: Is cadmium dangerous to human health?
A: Yes, cadmium is considered toxic to humans. Prolonged exposure to high levels of cadmium can lead to serious health issues, particularly affecting the kidneys, respiratory system, and potentially causing cancer.
Q: Are there any regulations or restrictions on cadmium use?
A: Yes, many countries have regulations and restrictions in place regarding the use of cadmium. These regulations focus on limiting cadmium exposure in the workplace, controlling its use in consumer products, and managing its disposal to prevent environmental contamination.
Q: Can cadmium be recycled?
A: Yes, cadmium can be recycled. It can be extracted from various waste sources, such as spent nickel-cadmium batteries, and recycled for reuse. Recycling cadmium helps reduce the environmental impact and conserves valuable resources.
Q: What are the environmental concerns associated with cadmium?
A: Cadmium pollution is a significant environmental concern. Improper disposal of cadmium-containing products and industrial waste can lead to soil and water contamination, posing risks to ecosystems and human health.
Q: Are there any alternative materials to cadmium in its various applications?
A: In certain applications, such as batteries and pigments, alternative materials are being explored to reduce reliance on cadmium. For example, nickel-metal hydride (NiMH) batteries are used as an alternative to nickel-cadmium (NiCd) batteries.
Q: How can individuals minimize their exposure to cadmium?
A: To minimize exposure to cadmium, individuals should avoid smoking, as tobacco smoke is a significant source of cadmium. Additionally, being aware of potential sources of cadmium, such as certain foods and contaminated water, and following proper safety measures in workplaces where cadmium is used are essential.












