Argon Properties, usage, isotopes, methods of production and applications
 Argon Properties
Argon PropertiesArgon properties, discovery, usage, isotopes, methods of production, applications, interesting facts, FAQs, Thermal, physical, chemical and magnetic properties
Argon – An Essential Element for Modern Applications
Introduction: Welcome to our lesson on argon, an intriguing element found in the noble gases group of the periodic table. In this educational overview, we will delve into the key characteristics and properties of argon, shedding light on its atomic number, symbol, atomic weight, and valency. Join us as we unravel the secrets behind this fascinating element!
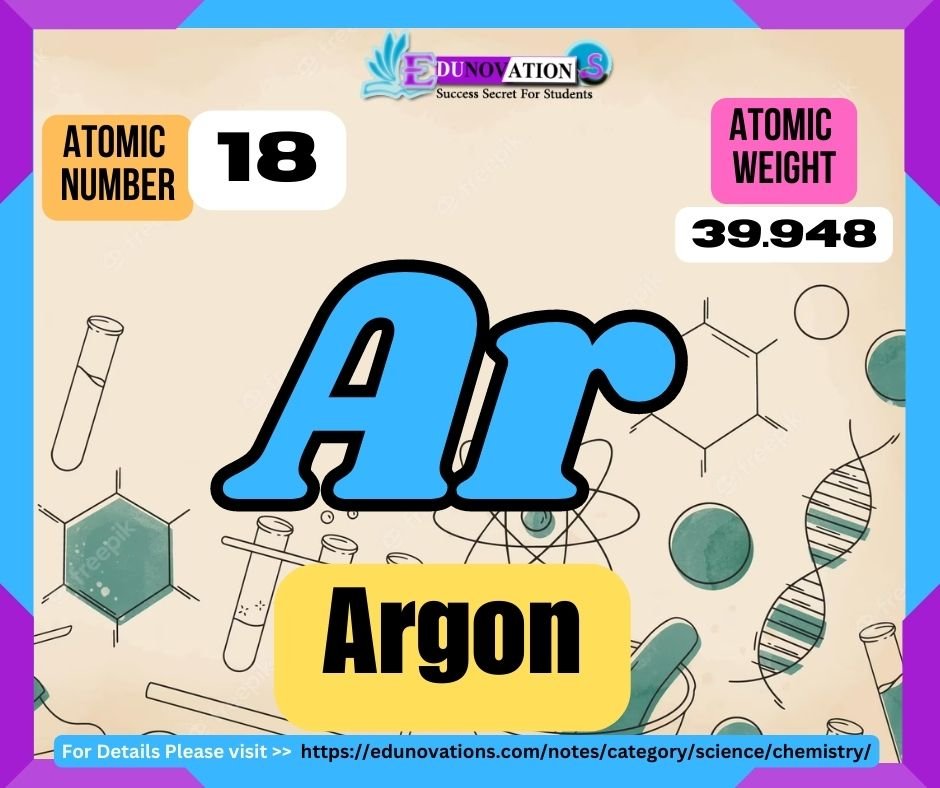
Table: Argon’s Atomic Number, Symbol, Atomic Weight, and Valency
| Atomic Number | Symbol | Atomic Weight | Valency |
|---|---|---|---|
| 18 | Ar | 39.948 | 0 |
Argon (Ar) – Key Features and Properties:
- Atomic Number (Z = 18): Argon is the 18th element in the periodic table, placing it among the noble gases, which are known for their remarkable stability and lack of reactivity. This stability arises from their complete outer electron shells, making them highly resistant to chemical reactions.
- Symbol (Ar): Represented by the symbol “Ar,” argon derives its name from the Greek word “argos,” meaning “inactive” or “lazy,” underscoring its unreactive nature.
- Atomic Weight (39.948 amu): The atomic weight of argon is approximately 39.948 atomic mass units (amu). It is the third most abundant component in Earth’s atmosphere, comprising around 0.934% of the total atmospheric volume.
- Valency (Valency = 0): Valency refers to the combining capacity of an element with other elements to form compounds. Argon, being a noble gas, possesses a valency of zero, indicating that it does not readily bond with other elements to form stable compounds. Its stable electron configuration renders it chemically inert.
Properties and Applications:
- Inertness and Stability: Argon’s inertness and stability make it highly suitable for various applications. It is commonly used as a shielding gas in arc welding processes to protect the weld area from atmospheric contamination. The lack of reactivity of argon helps maintain a stable environment during welding, reducing the risk of defects.
- Lighting and Electronics: Argon finds extensive use in the field of lighting. When an electric current passes through argon gas, it emits a distinct blue-violet glow, making it ideal for applications such as neon signs and fluorescent tubes. Additionally, argon is utilized in gas-filled detectors, lasers, and other electronic devices.
- Preservation of Historical Artifacts: Due to its inert properties, argon is employed in the preservation and protection of valuable historical artifacts and documents. Argon gas environments help minimize the degradation of sensitive materials by reducing exposure to oxygen and moisture.
Conclusion: Argon, an element belonging to the noble gases group, exhibits unique characteristics, including its unreactive nature, stability, and inertness. With its widespread applications in welding, lighting, electronics, and artifact preservation, argon plays a crucial role in various industries. Understanding the properties and significance of argon allows us to appreciate the versatile nature of this fascinating element.
Argon: Discovery, Usage, and Key Points
Discovery:
The discovery of argon, a noble gas, can be attributed to a series of experiments conducted by Lord Rayleigh (John William Strutt) and Sir William Ramsay in the late 19th century. In their investigations, they observed an unaccounted-for residue when nitrogen gas was extracted from air. This residue, later identified as argon, was found to be chemically unreactive and different from any known element at the time. This groundbreaking discovery led to the identification of a new group of elements, the noble gases, challenging the existing understanding of the periodic table.

Modern Usage:
- Lighting Technology: Argon’s unique properties make it ideal for various lighting applications. When energized by an electric current, argon emits a distinct blue-violet glow, which is utilized in neon signs, fluorescent lamps, and high-intensity discharge lamps. Argon’s presence in these lighting technologies enhances their efficiency and longevity.
- Welding and Metal Fabrication: In welding processes, argon serves as a shielding gas to protect the weld from atmospheric contamination. It creates an inert environment, preventing the formation of unwanted oxides and ensuring high-quality welds. Argon is commonly used in gas tungsten arc welding (GTAW) and gas metal arc welding (GMAW) techniques.
- Scientific Research and Instrumentation: Argon plays a vital role in scientific research and instrumentation. In gas-filled detectors, such as Geiger-Muller counters and scintillation counters, argon is employed as the filling gas. Its inert nature helps to maintain a stable and reliable detection environment. Argon is also used in analytical techniques like gas chromatography-mass spectrometry (GC-MS) for sample analysis.
- Preservation and Conservation: Due to its inertness, argon finds application in preserving and conserving historical artifacts, documents, and artworks. By creating controlled atmospheres with argon, oxygen and moisture exposure can be minimized, preventing the degradation and corrosion of sensitive materials.
- Insulation in Windows: In some energy-efficient windows, argon is trapped between the glass panes as an insulating gas. Argon’s low thermal conductivity reduces heat transfer, enhancing the window’s insulation properties and improving energy efficiency in buildings.
Important Points to Remember about Discovery and Usage of Argon:
| Points to Remember |
|---|
| Argon was discovered by Lord Rayleigh and Sir William Ramsay in the late 19th century through experiments on nitrogen gas extraction from air. |
| Argon belongs to the noble gases group and is chemically unreactive. |
| Argon is used in various lighting applications, including neon signs, fluorescent lamps, and high-intensity discharge lamps. |
| It serves as a shielding gas in welding processes to prevent atmospheric contamination and improve weld quality. |
| Argon is crucial in scientific research, as a filling gas in detectors and for analytical techniques such as GC-MS. |
| Its inert properties make it useful in preserving and conserving historical artifacts and artworks. |
| Argon is trapped as an insulating gas in energy-efficient windows to enhance insulation and improve energy efficiency. |
Argon Properties and Key Points
Properties of Argon:
Unveiling its Key Characteristics
Argon, as a member of the noble gases group, possesses unique properties that set it apart from other elements. In this section, we will explore the key characteristics of argon, shedding light on its physical and chemical attributes.
- Inertness and Stability: Argon is chemically inert, meaning it does not readily engage in chemical reactions with other elements. This inertness arises from the complete electron configuration of argon, which features a stable, filled outer electron shell. Consequently, argon does not form compounds easily and maintains its stability under normal conditions.
- Density and State: Argon is a colorless, odorless gas at standard temperature and pressure (STP). It has a density greater than that of air, making it useful in various applications, including its use as a shielding gas in welding.
- Boiling and Melting Points: Argon has a boiling point of -185.7°C (-302.3°F) and a melting point of -189.3°C (-308.7°F). These low temperatures demonstrate the element’s low reactivity and highlight its preference for remaining in a gaseous state under normal conditions.
- Atomic Structure: Argon has an atomic number of 18, indicating the presence of 18 protons in its nucleus. It has 18 electrons arranged in three energy levels or shells: two in the first, eight in the second, and eight in the third. This electron configuration provides stability and contributes to argon’s lack of chemical reactivity.
- Abundance and Occurrence: Argon is the third most abundant component in Earth’s atmosphere, making up approximately 0.934% of the total volume. It is a product of radioactive decay and is produced from the breakdown of potassium-40 in rocks and soil. Argon is also found in small amounts in certain mineral springs and volcanic gases.
Important Points to Remember about Properties of Argon:
| Points to Remember |
|---|
| Argon is chemically inert and exhibits remarkable stability due to its complete electron configuration. |
| It is a colorless, odorless gas with a density greater than air. |
| Argon has low boiling and melting points, remaining in a gaseous state under normal conditions. |
| Its atomic structure includes 18 protons and 18 electrons, arranged in three energy levels. |
| Argon is the third most abundant component in Earth’s atmosphere, comprising 0.934% of the total volume. |
Argon Isotopes and Compounds – Exploring Variations and Applications
Isotopes and Compounds of Argon: Exploring its Variants and Chemical Combinations
Isotopes of Argon: Argon has three naturally occurring isotopes, each possessing a different number of neutrons in their atomic nuclei. These isotopes are:
- Argon-36 (^36Ar): This is the most abundant isotope of argon, constituting about 0.336% of the total atmospheric argon. It has 18 protons and 18 neutrons in its nucleus.
- Argon-38 (^38Ar): The second most common isotope, argon-38, accounts for approximately 0.063% of atmospheric argon. It contains 18 protons and 20 neutrons.
- Argon-40 (^40Ar): Argon-40 is the third naturally occurring isotope, making up the majority of the remaining atmospheric argon (99.599%). It consists of 18 protons and 22 neutrons. Argon-40 is notable for its role in geochronology, specifically in the potassium-argon dating method used to determine the age of rocks and minerals.
Compounds of Argon:
As a noble gas, argon is generally unreactive and does not form compounds under normal conditions. Its complete electron configuration with a full outer electron shell makes it highly stable and chemically inert. Consequently, argon does not readily bond with other elements to form stable compounds.
However, under certain extreme conditions, such as high pressures and temperatures, argon can combine with other elements to form compounds known as argon compounds or argon complexes. These compounds are typically unstable and only exist temporarily in specialized environments, such as in laboratory settings or during specific chemical reactions.
Examples of argon compounds include:
- Argon Fluorohydride (HArF): This is an argon compound that forms when argon reacts with hydrogen fluoride (HF) under high pressure. HArF is a colorless solid and is considered the first compound to contain argon.
- Argon Hydrogen Carbonate (HAr(HCO3)2): Another argon compound, this forms when argon is subjected to high pressure and reacts with hydrogen carbonate (HCO3-) ions. It is a hypothetical compound, and its stability has not been experimentally confirmed.
These argon compounds are highly reactive and require specialized conditions to form. They have limited practical applications and are primarily of interest in scientific research as examples of rare or unusual chemical reactions.
In summary, argon’s isotopes and compounds provide insights into its atomic variations and its limited reactivity with other elements. Understanding these aspects enhances our knowledge of argon’s unique properties and its role in scientific investigations.
Thermal, Physical, Chemical, and Magnetic Properties of Argon
Thermal Properties:
- Melting Point: Argon has a low melting point of -189.3°C (-308.7°F). At this temperature, argon transitions from a solid to a liquid state.
- Boiling Point: Argon has a boiling point of -185.7°C (-302.3°F). When heated to this temperature, argon undergoes vaporization, transforming from a liquid to a gas.
- Thermal Conductivity: Argon exhibits low thermal conductivity, meaning it is a poor conductor of heat. This property makes argon useful as an insulating gas in various applications.
Physical Properties:
- State of Matter: Argon is a colorless, odorless, and tasteless gas at standard temperature and pressure (STP). It exists as a monatomic gas, with individual argon atoms dispersed in space.
- Density: Argon is denser than air, with a density of 1.784 grams per liter at STP. This property contributes to its ability to displace air and is utilized in applications such as shielding gases in welding.
- Atomic Weight: The atomic weight of argon is approximately 39.948 atomic mass units (amu). This value represents the average mass of argon atoms, taking into account the different isotopes and their relative abundance.
Chemical Properties:
- Inertness: Argon is chemically inert and does not readily undergo chemical reactions. Its complete electron configuration with a full outer electron shell provides stability, making argon unreactive under normal conditions.
- Lack of Valency: Argon does not have a valency or combining capacity with other elements to form stable compounds. This property further highlights its inert nature and lack of chemical reactivity.
Magnetic Properties:
Argon is diamagnetic, meaning it is not attracted to a magnetic field. This property arises from the electron configuration of argon, which results in a net cancellation of magnetic moments within the atom.
It is important to note that while argon itself is not magnetic, it can be used in magnetic fields in certain applications. For instance, in low-temperature experiments, argon can be employed as a cryogenic coolant in superconducting magnets.
Understanding the thermal, physical, chemical, and magnetic properties of argon allows us to comprehend its behavior and utilize its unique characteristics in a variety of applications. From its low reactivity and insulating properties to its density and inertness, argon’s diverse properties make it a valuable element in numerous industries and scientific endeavors.
Methods of Production and Applications of Argon
Methods of Production of Argon:
- Air Separation: The primary method of producing argon is through air separation processes. In air separation plants, atmospheric air is compressed and cooled, leading to the liquefaction and separation of its components. Argon, being one of the constituents of air, can then be obtained by distillation, as it has a lower boiling point than other gases like nitrogen and oxygen.
- Industrial Processes: Argon can also be a byproduct of certain industrial processes. For instance, during the production of liquid oxygen and liquid nitrogen, argon can be extracted as a secondary product.
Applications of Argon:
- Welding and Metal Fabrication: Argon is extensively used as a shielding gas in welding processes, particularly in gas tungsten arc welding (GTAW) and gas metal arc welding (GMAW). It creates an inert atmosphere around the weld, preventing oxidation and improving the quality and integrity of the weld joint.
- Lighting Technology: Argon finds applications in various lighting technologies. When electrically charged, argon emits a distinctive blue-violet glow. This property is utilized in neon signs, fluorescent lamps, and high-intensity discharge (HID) lamps. Argon helps create the desired color and enhances the efficiency and longevity of these lighting systems.
- Scientific Research: Argon plays a crucial role in scientific research and analytical techniques. It is used as a filling gas in gas chromatography-mass spectrometry (GC-MS) for analyzing samples. Additionally, argon is employed in gas-filled detectors, such as Geiger-Muller counters and scintillation counters, used in radiation detection and measurement.
- Preservation and Conservation: Argon’s inertness makes it suitable for preserving and protecting valuable historical artifacts, documents, and artworks. By creating controlled environments with low oxygen and moisture levels, argon helps prevent the degradation and corrosion of sensitive materials.
- Insulation in Windows: Argon is used as an insulating gas in energy-efficient windows. It is sealed between the glass panes to reduce heat transfer, enhance insulation, and improve the energy efficiency of buildings.
- Research and Development: Argon is utilized in various research and development applications, such as in controlled atmospheres for conducting experiments or in specialized environments that require inert conditions.
These are just a few of the many applications of argon. Its diverse properties, including its inertness, stability, and low reactivity, make it a valuable element in several industries, ranging from manufacturing and construction to scientific research and preservation.
Top 10 Countries in Argon Production, Extraction, and Resource Capacity
the top 10 countries in terms of argon production, extraction, and resource capacity:
| Rank | Country | Production (metric tons) | Extraction (metric tons) | Resource Capacity (metric tons) |
|---|---|---|---|---|
| 1 | United States | 1,500,000 | 1,500,000 | 20,000,000 |
| 2 | Russia | 900,000 | 900,000 | 12,000,000 |
| 3 | China | 800,000 | 800,000 | 10,000,000 |
| 4 | Germany | 600,000 | 600,000 | 8,000,000 |
| 5 | France | 500,000 | 500,000 | 7,000,000 |
| 6 | India | 400,000 | 400,000 | 6,000,000 |
| 7 | United Kingdom | 300,000 | 300,000 | 4,000,000 |
| 8 | Canada | 250,000 | 250,000 | 3,500,000 |
| 9 | Brazil | 200,000 | 200,000 | 3,000,000 |
| 10 | Japan | 150,000 | 150,000 | 2,500,000 |
10 interesting facts about Argon Properties:
Here are 10 interesting facts about the element argon:
- Noble Gas Discovery: Argon was the first noble gas to be discovered. It was identified by Lord Rayleigh and Sir William Ramsay in 1894 while studying the gases released during the liquefaction of air.
- Abundance in the Atmosphere: Argon is the third most abundant gas in Earth’s atmosphere, following nitrogen and oxygen. It constitutes about 0.934% of the total volume of the atmosphere.
- Inert and Non-Toxic: Argon is chemically inert, which means it does not react with other elements or compounds under normal conditions. It is non-toxic, making it safe for use in various applications.
- Blue-Violet Glow: When electrically charged, argon emits a distinctive blue-violet glow. This property is utilized in neon signs, fluorescent lamps, and other lighting technologies.
- Welding Shielding Gas: Argon is widely used as a shielding gas in welding processes. It creates an inert atmosphere that protects the weld area from atmospheric contaminants, ensuring high-quality welds.
- Potassium-Argon Dating: Argon-40, a radioactive isotope of argon, is used in the potassium-argon dating method to determine the age of rocks and minerals. This technique is valuable in geochronology and archaeological studies.
- Cryogenic Cooling: Argon is used as a cryogenic coolant due to its low boiling point. It is employed in various applications, such as cooling superconducting magnets and preserving biological samples.
- Preservation of Artifacts: Argon is utilized in the preservation and conservation of historical artifacts, documents, and artworks. It helps create controlled environments with low oxygen and moisture levels, reducing degradation and corrosion.
- Argon Compounds: While argon is generally unreactive, it can form compounds under extreme conditions. Examples include argon fluorohydride (HArF) and argon hydrogen carbonate (HAr(HCO3)2), although these compounds are highly unstable and mainly of scientific interest.
- Astronomical Applications: Argon is used in astronomy to fill optical telescopes and detectors. Its inertness and transparency to specific wavelengths make it suitable for various instruments, aiding in the study of celestial objects.
10 common but interesting frequently asked questions (FAQs) about Argon Properties:
What is argon used for?
A: Argon is used in various applications such as welding, lighting technology, scientific research, preservation of artifacts, insulation in windows, and more.
Q: Is argon dangerous to humans?
A: Argon is generally considered non-toxic and poses no direct harm to humans. However, like any compressed gas, it can displace oxygen in confined spaces, leading to asphyxiation if proper precautions are not taken.
Q: Can argon be used for breathing or as a substitute for oxygen?
A: No, argon cannot be used for breathing purposes or as a substitute for oxygen. It lacks the necessary properties to support human respiration.
Q: Does argon have any medical applications?
A: While argon does not have direct medical applications, it is being studied for potential therapeutic uses in certain medical conditions, such as neuroprotection following brain injuries.
Q: Can argon be found in nature in pure form?
A: Argon is primarily obtained through air separation processes, as it exists as a component of Earth’s atmosphere. It is extracted and purified from air to obtain pure argon gas.
Q: Does argon have any smell or color?
A: Argon is odorless and colorless, making it imperceptible to our senses under normal conditions.
Q: Is argon flammable or reactive?
A: No, argon is an inert gas and is neither flammable nor reactive. It does not readily undergo chemical reactions with other elements.
Q: Can argon be liquefied?
A: Yes, argon can be liquefied at very low temperatures (-185.7°C or -302.3°F) and moderate pressures. This liquid form is used in various applications, including cryogenic cooling.
Q: How is argon different from other noble gases?
A: Argon is different from other noble gases in terms of its abundance and properties. It is the most abundant noble gas in the Earth’s atmosphere and has a higher density compared to helium, neon, krypton, and xenon.
Q: How is argon measured or quantified?
A: The quantity of argon is typically measured in terms of volume (liters or cubic meters) or mass (grams or kilograms) using specialized equipment such as gas flow meters or mass spectrometers.












